Abstract
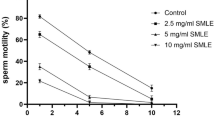
Human fertility regulation is a major way to control overpopulation. In this perspective, this study emphasized the in vitro effect of hydro-methanol extract of Tinospora cordifolia (TCHME) stem for spermicidal and reproductive hypo-functions using human and rat samples. Control, 0.5-, 1-, and 2-mg TCHME-charged groups were considered to assess the relevant parameters. Levels of spermiological parameters like sperm motility, viability, the integrity of plasma and acrosomal membrane, and nuclear chromatin decondensation were significantly reduced (p < 0.05) in the dose- and duration-dependent TCHME-charged groups compared to the control. The inhibitory concentration 50 (IC50) of TCHME on motile human and rat sperms were 0.8 and 0.4 mg/ml, respectively. Testicular androgenic key enzymes and antioxidant enzymes (human sperm pellet, testes, and epididymis of rat)’ activities were significantly diminished (p < 0.05), while antioxidant enzymes’ activities were significantly elevated (p < 0.05) in renal and insignificantly (p > 0.05) elevated in hepatic tissues of rat in TCHME-charged groups compared to the control. Significant elevation (p < 0.05) of thiobarbituric acid reactive substances (TBARS)’ level in human sperm pellet, testes, and epididymis of rats and significant diminution (p < 0.05) in TBARS levels of liver and kidney were observed in TCHME-charged groups. It focused that TCHME is more potent for stress imposition on reproductive tissues and sperm compared to the other tested tissues. Non-significant alterations (p > 0.05) in glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) activities in the said organs of rat indicated its non-toxic effect. It highlighted that TCHME possesses spermicidal and reproductive tissue-specific effects which strengthen the possibilities of male contraceptive development from it.






Similar content being viewed by others
Data availability
Authors can show the data of the study if there is any valid request from the editorial board of the journal.
Code Availability
Software Origin Lab, Version 8.5 for Windows 10.
References
Chappell BT, Brooke LG, Brandon H. Mechanisms of action of currently available woman-controlled, vaginally administered, non-hormonal contraceptive products. Ther Adv Reprod Health. 2022;16:1–6. https://doi.org/10.1177/11795581211107120.
Xu M, Zhao M, Li RHW, Lin Z, Chung JPW, Li TC, Lee TL, Chan DYL. Effects of nonoxynol-9 (N-9) on sperm functions: systematic review and meta-analysis. Reprod Fertil. 2022;3(1):R19–33. https://doi.org/10.1530/RAF-21-0024.
D’Cruz OJ, Erbeck D, Uckun FM. A study of the potential of the pig as a model for the vaginal irritancy of benzalkonium chloride in comparison to the nonirritant microbicide PHI-443 and the spermicide vanadocene dithiocarbamate. Toxicol Pathol. 2005;33(4):465–76. https://doi.org/10.1080/01926230590959866.
Ghaffarilaleh V, Fisher D, Henkel R. Carica papaya seed extract slows human sperm. J Ethnopharmacol. 2019;241:111972. https://doi.org/10.1016/j.jep.2019.111972.
Sharma SK, SaiRam M, Ilavazhagan G, Devendra K, Shivaji SS, Selvamurthy W. Mechanism of action of NIM-76: a novel vaginal contraceptive from neem oil. Contraception. 1996;54(6):373–8. https://doi.org/10.1016/s0010-7824(96)00204-1.
Paul D, De D, Ali KM, Chatterjee K, Nandi DK, Ghosh D. Comparative study on the spermicidal activity of organic solvent fractions from hydroethanolic extracts of Achyranthes aspera and Stephania hernandifolia in human and rat sperm. Contraception. 2010;81(4):355–61. https://doi.org/10.1016/j.contraception.2009.09.001.
Anand AK, Prasad V, Alam M. Herbal or modern methods of contraception! Choice is yours. Int J Reprod Contracept Obstet Gynecol. 2015;4(4):947–53. https://doi.org/10.18203/2320-1770.ijrcog20150405.
UN Population Division. World population prospects 2019. In: Department of Economic and Social Affairs. 2019. Retrieved from http://www.ncbi.nlm. nih.gov/pubmed/12283219. Accessed 04.09.2022.
Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF, Tang MK, Sun JN, Ma DL, Han YF, Fong WF, Ko KM. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med. 2013;2013:1–25. https://doi.org/10.1155/2013/627375.
Bhatt N, Deshpande M. A Critical review and scientific prospective on contraceptive therapeutics from ayurveda and allied ancient knowledge. Front Pharmacol. 2021;12:629591. https://doi.org/10.3389/fphar.2021.629591.
Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) – validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1(2):112–21. https://doi.org/10.4103/0974-7788.64405.
Gupta RS, Sharma A. Antifertility effect of Tinospora cordifolia (Willd.) stem extract in male rats. Indian J Exp Biol. 2003;41(8):885–9.
Chaudhury K, Bhattacharyya AK, Guha SK. Studies on the membrane integrity of human sperm treated with a new injectable male contraceptive. Hum Reprod. 2004;19(8):1826–30. https://doi.org/10.1093/humrep/deh332.
Zemjanis R. Diagnostic and therapeutic techniques in animal reproduction. 2nd ed. Baltimore: the Williams & Wilkins Company; 1970. p. 88–96.
Ratnasooriya WD, Amarasekera AS, Perera NSD, Premakumara GAS. Sperm antimotility properties of a seed extract of Abrus precatorius. J Ethnopharmacol. 1991;33(1-2):85–90. https://doi.org/10.1016/0378-8741(91)90166-B.
World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 5th ed. Geneva: World Health Organization press. New York, NY: Cambridge University Press; 2010. p. 7–271.
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. Reproduction. 1984;70(1):219–28. https://doi.org/10.1530/jrf.0.0700219.
Gopalkrishnan K. Standardized procedures in human semen analysis. Curr Sci. 1995;68(4):353–62. https://www.jstor.org/stable/24096433
Gopalkrishnan K, Hinduja IN, Anand Kumar TC. In vitro decondensation of nuclear chromatin of human spermatozoa: assessing fertilizing potential. Arch Androl. 1991;27(1):43–50. https://doi.org/10.3109/01485019108987650.
Jarabak J, Adams JA, Williams-Ashman HG, Talalay P. Purification of 17β-hydroxysteroid dehydrogenase of human placenta and studies on its trans dehydrogenase function. J Biol Chem. 1962;237(2):345–57.
Talalay P. Hydroxysteroid dehydrogenase. In: Colowic S, editor. Methods in Enzymology. New York, NY: Academic Press; 1962. p. 512–6.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–40.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. https://doi.org/10.1016/0003-2697(79)90738-3.
Jagadeesan G, Kavitha AV. Recovery of phosphatase and transaminase activity of mercury intoxicated Mus musculus (Linn.) liver tissue by Tribulus terrestris (Linn.) (Zygophyllaceae) extract. Trop Biomed. 2006;23(1):45–51.
Hossain MA, KAS AL-R, ZH AL-M, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–10. https://doi.org/10.1016/S2221-1691(13)60142-2.
Gauri SS, Mandal SM, Atta S, Dey S, Pati BR. Novel route of tannic acid biotransformation and their effect on major biopolymer synthesis in Azotobacter sp. SSB81. J App Microbiol. 2013;114(1):84–95. https://doi.org/10.1111/jam.12030.
Li M, Wang H, Huan X, Cao N, Guan H, Zhang H, Cheng X, Wang C. Simultaneous LC-MS/MS bioanalysis of alkaloids, terpenoids, and flavonoids in rat plasma through salting-out-assisted liquid-liquid extraction after oral administration of extract from Tetradium ruticarpum and Glycyrrhiza uralensis: A sample preparation strategy to broaden analyte coverage of herbal medicines. Anal Bioanal Chem. 2021;2021(413):5871–84. https://doi.org/10.1007/s00216-021-03568-1.
Chan PJ, Corselli JU, Jacobson JD, Patton WC, King A. Spermac stain analysis of human sperm acrosomes. Fertil Steril. 1999;72(1):124–8. https://doi.org/10.1016/s00150282(99)00201-0.
Bajpai V, Singh A, Chandra P, Negi MPS, Kumar N, Kumar B. Analysis of phytochemical variations in dioecious Tinospora cordifolia stems using HPLC/QTOF MS/MS and UPLC/QqQLIT-MS/MS. Phytochem Anal. 2016;27(2):92–9. https://doi.org/10.1002/pca.2601.
Goufo P, Singh RK, Cortez I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants. 2020;9(5):398. https://doi.org/10.3390/antiox9050398.
Sefrioui MR, El Othmani IS, Filali H, Derfoufi S, Derraji S, Benmoussa A, Said AAH. Evaluation of spermicidal activity of saponosides from Saponaria officinalis/Caryophyllaceae, Glycyrrhizia glabra/Fabaceae and Herniaria glabra/Caryophyllaceae. Med Pharm Rep. 2021;94(2):239–47. https://doi.org/10.15386/mpr-1879.
Okon UA, Etim BN. Citrus aurantifolia impairs fertility facilitators and indices in male albino wistar rats. Int J Reprod Contracept Obstet Gynecol. 2014;3(3):640–6. https://doi.org/10.5455/2320-1770.ijrcog20140949.
Davila MP, Muñoz PM, Bolaños JG, Stout TAE, Gadella BM, Tapia JA, Silva CB, Ferrusola CO, Pena FJ. Mitochondrial ATP is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction. 2016;152(6):683–94. https://doi.org/10.1530/REP-16-0409.
Bennison C, Hemmings N, Brookes L, Slate J, Birkhead T. Sperm morphology, adenosine triphosphate (ATP) concentration and swimming velocity: unexpected relationships in a passerine bird. Proc Biol Sci. 2016;283(1837):20161558. https://doi.org/10.1098/rspb.2016.1558.
Castellini C, D’Andrea S, Cordeschi G, Totaro M, Parisi A, Di Emidio G, Tatone C, Francavilla S, Barbonetti A. Pathophysiology of mitochondrial dysfunction in human spermatozoa: focus on energetic metabolism, oxidative stress and apoptosis. Antioxidants. 2021;10(5):695. https://doi.org/10.3390/antiox10050695.
Walczak–Jedrzejowska R, Wolski JK, Slowikowska–Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66(1):60–7. https://doi.org/10.5173/ceju.2013.01.art19.
Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22(9):1–21. https://doi.org/10.3390/ijms022094642.
Shan S, Xu F, Hirschfeld M, Brenig B. Sperm lipid markers of male fertility in mammals. Int J Mol Sci. 2021;22(16):1–21. https://doi.org/10.3390/ijms22168767.
Chakrabarti K, Pal S, Bhattacharyya AK. Sperm immobilization activity of Allium sativum L. and other plant extracts. Asian J Androl. 2003;5(2):131–6.
Ribas-Maynou J, Garcia-Bonavila E, Hidalgo CO, Catalán J, Miró J, Yeste M. Species-specific differences in sperm chromatin decondensation between eutherian mammals underlie distinct lysis requirements. Front Cell Dev Biol. 2021;9:669182. https://doi.org/10.3389/fcell.2021.669182.
Mondal P, Maity R, Mallick C. In vitro spermicidal effect of Thevetia peruviana leaves on human spermatozoa. Andrologia. 2022;54(2):e14323. https://doi.org/10.1111/and.14323.
Gervasi MG, Visconti PE. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology. 2017;5(2):204–18. https://doi.org/10.1111/andr.12320.
Guido C, Santoro M, De Amicis F, Perrotta I, Panza S, Rago V, Cesario MG, Lanzino M, Aquila S. Human sperm anatomy and endocrinology in varicocele: role of androgen receptor. Reproduction. 2014;147(5):589–98. https://doi.org/10.1530/REP-13-0542.
Hu GX, Zhao BH, Chu YH, Zhou HY, Akingbemi BT, Zheng ZQ, Ge RS. Effects of genistein and equol on human and rat testicular 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase 3 activities. Asian J Androl. 2010;12(4):519–26. https://doi.org/10.1038/aja.2010.18.
Salahudeen MS, Nishtala PS. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm J. 2017;25(2):165–75. https://doi.org/10.1016/j.jsps.2016.07.002.
Acknowledgements
The authors are grateful to the UGC for providing the funding necessary to complete this work. University Grants Commission (UGC), Govt. of India. NTA Ref No.: 200510431209.
Author information
Authors and Affiliations
Contributions
Puja Das conducted the project, methodology, and drafted the original manuscript. Data analysis and data curation were performed by Kuladip Jana, and Dipanwita Mitra handled the animals and collected the human semen samples. Debidas Ghosh supervised, conceptualized the study design, and edited and validated the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Ethics Committee of Vidyasagar University with reference no. VU/IAEC/CPCSEA/6/7/2022 dt.22.11.2022.
Consent to participate
Each participant signed the informed consent.
Consent for publication
All authors agree to publish the article in this journal after acceptance.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, P., Mitra, D., Jana, K. et al. In Vitro Study on Spermicidal Action of Hydro-methanol Extract of Tinospora cordifolia (Willd.) Stem in Rat and Human Sperm: a Comparative Analysis. Reprod. Sci. 30, 3480–3494 (2023). https://doi.org/10.1007/s43032-023-01327-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01327-4