Abstract
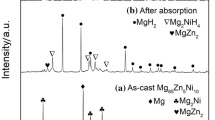
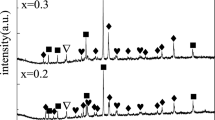
Mg81Ni19-8wt.% REO (oxides of Lanthanum and Cerium) alloys were successfully prepared using mechanical alloying method with Mg-Ni alloy and REO powder. Phase analysis, structural characterization, and microstructure imagine of the alloys were conducted using X-ray diffraction (XRD), metallurgical microscope, and transmission electron microscopy (TEM) methods. Multi-phase structures, including the primary phase of Mg2Ni and several secondary phases of Mg + Mg2Ni, MgNi-LaO, and MgNi-CeO, were found in in the as-cast Mg81Ni19-8wt.% REO alloys. XRD and TEM results showed that Ce exhibits variable valence behavior at various stages, and the addition of REO promotes the nanocrystalline of the alloy. The hydrogen absorption capacity of ball-milled Mg81Ni19 and Mg81Ni19-8wt.%REO alloy for 2 h at 343 K is 1.34 wt.% and 1.83 wt.%, which are much larger than 0.94 wt.% of as-cast Mg81Ni19 alloy. The addition of REO led to a decrease of the thermal decomposition temperature of the alloy hydride by approximately 20 K and a reduction of the activation energy of the hydrogen desorption reaction by 10% and 13%, respectively.













Similar content being viewed by others
References
Panwar NL, Kaushik SC, Kothari S (2011) Role of renewable energy sources in environmental protection: a review. Renew Sustain Energy Rev 15:1513–1524. https://doi.org/10.1016/j.rser.2010.11.037
Dincer I (2000) Renewable energy and sustainable development: a crucial review. Renew Sustain Energy Rev 4:157–175. https://doi.org/10.1016/S1364-0321(99)00011-8
Ibrahim H, Ilinca A, Perron J (2008) Energy storage systems—characteristics and comparisons. Renew Sustain Energy Rev 12:1221–1250. https://doi.org/10.1016/j.rser.2007.01.023
Dutta S (2014) A review on production, storage of hydrogen and its utilization as an energy resource. J Ind Eng Chem 20:1148–1156. https://doi.org/10.1016/j.jiec.2013.07.037
Ipsakis D, Voutetakis S, Seferlis P, Stergiopoulos F, Elmasides C (2009) Power management strategies for a stand-alone power system using renewable energy sources and hydrogen storage. Int J Hydrog Energy 34:7081–7095. https://doi.org/10.1016/j.ijhydene.2008.06.051
Balat M, Balat M (2009) Political, economic and environmental impacts of biomass-based hydrogen. Int J Hydrog Energy 34:3589–3603. https://doi.org/10.1016/j.ijhydene.2009.02.067
Mori D, Hirose K (2009) Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int J Hydrog Energy 34:4569–4574. https://doi.org/10.1016/j.ijhydene.2008.07.115
Gielen D, Boshell F, Saygin D, Bazilian MD, Wagner N, Gorini R (2019) The role of renewable energy in the global energy transformation. Energy Strategy Rev 24:38–50. https://doi.org/10.1016/j.esr.2019.01.006
Yang M, Chen Y, Wang H, Zou Y, Wu P, Zou J, Jiang J (2022) Solvothermal preparation of CeO2 nanoparticles–graphene nanocomposites as an electrochemical sensor for sensitive detecting pentachlorophenol. Carbon Lett 32:1277–1285. https://doi.org/10.1007/s42823-022-00353-7
Chen Y, Tu C, Liu Y, Liu P, Gong P, Wu G, Jiang J (2023) Microstructure and mechanical properties of carbon graphite composites reinforced by carbon nanofibers. Carbon Lett 33:561–571. https://doi.org/10.1007/s42823-022-00445-4
Li F, Anjarsari Y, Wang J, Azzahiidah R, Jiang J, Zou J, Arramel MH (2022) Modulation of the lattice structure of 2D carbon-based materials for improving photo/electric properties. Carbon Lett. https://doi.org/10.1007/s42823-022-00380-4
Jacobson MZ (2009) Review of solutions to global warming, air pollution, and energy security. Energy Environ Sci 2:148–173. https://doi.org/10.1039/B809990C
Ball M, Wietschel M (2009) The future of hydrogen: opportunities and challenges☆. Int J Hydrog Energy 34:615–627. https://doi.org/10.1016/j.ijhydene.2008.11.014
Jiang J, Bai S, Yang M, Zou J, Li N, Peng J, Zhai T (2022) Strategic design and fabrication of MXenes-Ti3CNCl2@ CoS2 core-shell nanostructure for high-efficiency hydrogen evolution. Nano Res 15:5977–5986. https://doi.org/10.1007/s12274-022-4276-8
Zou J, Wu S, Liu Y, Sun Y, Cao Y, Hsu JP, Jiang J (2018) An ultra-sensitive electrochemical sensor based on 2D g-C3N4/CuO nanocomposites for dopamine detection. Carbon 130:652–663. https://doi.org/10.1016/j.carbon.2018.01.008
Abdalla AM, Hossain S, Nisfindy OB, Azad AT, Dawood M, Azad AK (2018) Hydrogen production, storage, transportation and key challenges with applications: a review. Energy Convers Manag 165:602–627. https://doi.org/10.1016/j.enconman.2018.03.088
Schlapbach L, Züttel A (2010) Hydrogen-storage materials for mobile applications. Mater Sustain Energy 414:353
Niaz S, Manzoor T, Pandith AH (2015) Hydrogen storage: materials, methods and perspectives. Renew Sustain Energy Rev 50:457–469. https://doi.org/10.1016/j.rser.2015.05.011
Bellosta von Colbe J, Ares J-R, Barale J, Baricco M, Buckley C, Capurso G, Gallandat N, Grant DM, Guzik MN, Jacob I, Jensen EH, Jensen T, Jepsen J, Klassen T, Lototskyy MV, Manickam K, Montone A, Puszkiel J, Sartori S, Sheppard DA, Stuart A, Walker G, Webb CJ, Yang H, Yartys V, Züttel A, Dornheim M (2019) Application of hydrides in hydrogen storage and compression: achievements, outlook and perspectives. Int J Hydrog Energy 44:7780–7808. https://doi.org/10.1016/j.ijhydene.2019.01.104
Hirscher M, Becher M (2003) Hydrogen storage in carbon nanotubes. J Nanosci Nanotechnol 3:3–17. https://doi.org/10.1166/jnn.2003.172
Qiao W, Yin D, Zhao S, Ding N, Liang L, Wang C, Wang L, He M, Cheng Y (2023) Effects of Cu do** on the hydrogen storage performance of Ti-Mn-based, AB2-type alloys. Chem Eng J 465:142837. https://doi.org/10.1016/j.cej.2023.142837
Zou J, Liao G (2022) In-situ construction of sulfur-doped g-C3N4/defective g-C3N4 Iso-type step-scheme heterojunction for boosting photocatalytic H2 evolution. Chin J Struct Chem 41:25–33. https://doi.org/10.14102/j.cnki.0254-5861.2021-0039
Sandrock G (1999) A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J Alloys Compd 293–295:877–888. https://doi.org/10.1016/S0925-8388(99)00384-9
Yartys VA, Lototsky MV (2004) An overview of hydrogen storage methods. In: Veziroglu TNY, Zaginaichenko S, Schur DV, Baranowski B, Shpak AP, Skorokhod VV (eds) Hydrogen materials science and chemistry of carbon nanomaterials. Springer, pp 75–104
Jiang J, Ou-yang L, Zhu L, Zheng A, Zou J, Yi X, Tang H (2014) Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: a study by Raman spectroscopy coupled with first-principles calculations. Carbon 80:213–221. https://doi.org/10.1016/j.carbon.2014.08.059
Bai S, Yang M, Jiang J, He X, Zou J, **ong Z, Liu S (2021) Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. npj 2D Mater Appl 5:78. https://doi.org/10.1038/s41699-021-00259-4
Boateng E, Chen A (2020) Recent advances in nanomaterial-based solid-state hydrogen storage. Mater Today Adv 6:100022. https://doi.org/10.1016/j.mtadv.2019.100022
Deng F, Jiang J, Sirés I (2023) State-of-the-art review and bibliometric analysis on electro-Fenton process. Carbon Lett 33:17–34. https://doi.org/10.1007/s42823-022-00420-z
Wang J, Qin Q, Li F, Anjarsari Y, Sun W, Azzahiidah R, Zou J, **ang K, Ma H, Jiang J, Arramel (2022) Recent advances of MXenes Mo2C-based materials for efficient photocatalytic hydrogen evolution reaction. Carbon Lett. https://doi.org/10.1007/s42823-022-00401-2
Wang J, Wang G, Cheng B, Yu J, Fan J (2021) Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin J Catal 42:56–68. https://doi.org/10.1016/j.jmst.2021.12.018
Hanada N, Ichikawa T, Fujii H (2005) Catalytic effect of nanoparticle 3D-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J Phys Chem B 109:7188–7194. https://doi.org/10.1021/jp044576c
Liang G, Huot J, Boily S, Van Neste A, Schulz R (1999) Hydrogen storage properties of the mechanically milled MgH2–V nanocomposite. J Alloys Compd 291:295–299. https://doi.org/10.1016/S0925-8388(99)00268-6
Abe JO, Popoola API, Ajenifuja E, Popoola OM (2019) Hydrogen energy, economy and storage: Review and recommendation. Int J Hydrog Energy 44:15072–15086. https://doi.org/10.1016/j.ijhydene.2019.04.068
Varin RA, Czujko T, Wronski ZS (2009) Nanomaterials for solid state hydrogen storage. Springer
Martin M, Gommel C, Borkhart C, Fromm E (1996) Absorption and desorption kinetics of hydrogen storage alloys. J Alloys Compd 238:193–201. https://doi.org/10.1016/0925-8388(96)02217-7
Wang H, Lin HJ, Cai WT, Ouyang LZ, Zhu M (2016) Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems: a review of recent progress. J Alloys Compd 658:280–300. https://doi.org/10.1016/j.jallcom.2015.10.090
Zhang Q, Zang L, Huang Y, Gao P, Jiao L, Yuan H, Wang Y (2017) Improved hydrogen storage properties of MgH2 with Ni-based compounds. Int J Hydrog Energy 42:24247–24255. https://doi.org/10.1016/j.ijhydene.2017.07.220
**e L, Li J, Zhang T, Song L, Kou H (2017) Microstructure and hydrogen storage properties of Mg-Ni-Ce alloys with a long-period stacking ordered phase. J Power Sources 338:91–102. https://doi.org/10.1016/j.jpowsour.2016.11.025
Oelerich W, Klassen T, Bormann R (2001) Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J Alloys Compd 315:237–242. https://doi.org/10.1016/S0925-8388(00)01284-6
Zhang C, Liang L, Zhao S, Wu Z, Wang S, Yin D, Wang Q, Wang L, Wang C, Cheng Y (2023) Dehydrogenation behavior and mechanism of LiAlH4 adding nano-CeO2 with different morphologies. Nano Res. https://doi.org/10.1007/s12274-023-5636-8
Pukazhselvan D, Capurso G, Maddalena A, Lo Russo S, Fagg DP (2014) Hydrogen storage characteristics of magnesium impregnated on the porous channels of activated charcoal scaffold. Int J Hydrog Energy 39:20045–20053. https://doi.org/10.1016/j.ijhydene.2014.10.038
Dornheim M, Doppiu S, Barkhordarian G, Boesenberg U, Klassen T, Gutfleisch O, Bormann R (2007) Hydrogen storage in magnesium-based hydrides and hydride composites. Scr Mater 56:841–846. https://doi.org/10.1016/j.scriptamat.2007.01.003
Aguey-Zinsou K-F, Ares-Fernández J-R (2010) Hydrogen in magnesium: new perspectives toward functional stores. Energy Environ Sci 3:526. https://doi.org/10.1039/b921645f
Zhang Y, Wei X, Zhang W, Yuan Z, Gao J, Ren H (2021) Catalytic effect comparison of TiO2 and La2O3 on hydrogen storage thermodynamics and kinetics of the as-milled La-Sm-Mg-Ni-based alloy. J Magnes Alloys 9:2063–2077. https://doi.org/10.1016/j.jma.2021.03.006
Du J, Lan Z, Zhang H, Lü S, Liu H, Guo J (2019) Catalytic enhanced hydrogen storage properties of Mg-based alloy by the addition of reduced graphene oxide supported V2O3 nanocomposite. J Alloys Compd 802:660–667. https://doi.org/10.1016/j.jallcom.2019.06.221
Chen B-H, Chuang Y-S, Chen C-K (2016) Improving the hydrogenation properties of MgH2 at room temperature by do** with nano-size ZrO2 catalyst. J Alloys Compd 655:21–27. https://doi.org/10.1016/j.jallcom.2015.09.163
Long S, Zou J, Chen X, Zeng X, Ding W (2014) A comparison study of Mg–Y2O3 and Mg–Y hydrogen storage composite powders prepared through arc plasma method. J Alloys Compd 615:S684–S688. https://doi.org/10.1016/j.jallcom.2013.11.159
Liu P, Chen H, Yu H, Liu X, Jiang R, Li X, Zhou S (2019) Oxygen vacancy in magnesium/cerium composite from ball milling for hydrogen storage improvement. Int J Hydrog Energy 44:13606–13612. https://doi.org/10.1016/j.ijhydene.2019.03.258
Zhang H, Fu L, Xuan W, Qi J (2020) Surface modification of the La1.7Mg1.3Ni9 alloy with trace Y2O3 related to the electrochemical hydrogen storage properties. Renew Energy 145:1572–1577. https://doi.org/10.1016/j.renene.2019.07.080
Zou J, Zeng X, Ying Y, Chen X, Guo H, Zhou S, Ding W (2013) Study on the hydrogen storage properties of core–shell structured Mg–RE (RE = Nd, Gd, Er) nano-composites synthesized through arc plasma method. Int J Hydrog Energy 38:2337–2346. https://doi.org/10.1016/j.ijhydene.2012.11.145
Long S, Zou J, Liu Y, Zeng X, Ding W (2013) Hydrogen storage properties of a Mg–Ce oxide nano-composite prepared through arc plasma method. J Alloys Compd 580:S167–S170. https://doi.org/10.1016/j.jallcom.2013.02.063
Zou J, Zeng X, Ying Y, Stephane P, Ding W (2012) Preparation and hydrogen sorption properties of a nano-structured Mg based Mg–La–O composite. Int J Hydrog Energy 37:13067–13073. https://doi.org/10.1016/j.ijhydene.2012.04.136
Zhang Y, Li Y, Shang H, Yuan Z, Qi Y, Dong X, Guo S (2018) Hydrogen storage performance of the as-milled Y Mg Ni alloy catalyzed by CeO2. Int J Hydrog Energy 43:1643–1650. https://doi.org/10.1016/j.ijhydene.2017.11.112
Wang F, Li R, Ding C, Wan J, Yu R, Wang Z (2016) Effect of catalytic Ni coating with different depositing time on the hydrogen storage properties of ZrCo alloy. Int J Hydrog Energy 41:17421–17432. https://doi.org/10.1016/j.ijhydene.2016.07.077
Peng D, Ding Z, Zhang L, Fu Y, Wang J, Li Y, Han S (2018) Remarkable hydrogen storage properties and mechanisms of the shell–core MgH2@carbon aerogel microspheres. Int J Hydrog Energy 43:3731–3740. https://doi.org/10.1016/j.ijhydene.2018.01.004
**ao X, Liu G, Peng S, Yu K, Li S, Chen C, Chen L (2010) Microstructure and hydrogen storage characteristics of nanocrystalline Mg+xwt% LaMg2Ni (x=0–30) composites. Int J Hydrog Energy 35:2786–2790. https://doi.org/10.1016/j.ijhydene.2009.05.024
Harrison JT, Pantoja E, Jang M-H, Gupta MC (2020) Laser sintered PbSe semiconductor thin films for Mid-IR applications using nanocrystals. J Alloys Compd 849:156537. https://doi.org/10.1016/j.jallcom.2020.156537
House SD, Vajo JJ, Ren C, Rockett AA, Robertson IM (2015) Effect of ball-milling duration and dehydrogenation on the morphology, microstructure and catalyst dispersion in Ni-catalyzed MgH2 hydrogen storage materials. Acta Mater 86:55–68. https://doi.org/10.1016/j.actamat.2014.11.047
Balcerzak M (2017) Structure and hydrogen storage properties of mechanically alloyed Ti-V alloys. Int J Hydrog Energy 42:23698–23707. https://doi.org/10.1016/j.ijhydene.2017.03.224
Mustafa NS, Ismail M (2017) Hydrogen sorption improvement of MgH2 catalyzed by CeO2 nanopowder. J Alloys Compd 695:2532–2538. https://doi.org/10.1016/j.jallcom.2016.11.158
Yong H, Guo S, Yuan Z, Zhang W, Qi Y, Zhao D, Zhang Y (2020) Phase evolution, hydrogen storage thermodynamics and kinetics of ternary Mg90Ce5Sm5 alloy. J Rare Earths 38:633–641. https://doi.org/10.1016/j.jre.2019.05.012
Paskevicius M, Sheppard DA, Buckley CE (2010) Thermodynamic changes in mechanochemically synthesized magnesium hydride nanoparticles. J Am Chem Soc 132:5077–5083. https://doi.org/10.1021/ja908398u
Liu H, He P, Feng JC, Cao J (2009) Kinetic study on nonisothermal dehydrogenation of TiH2 powders. Int J Hydrog Energy 34:3018–3025. https://doi.org/10.1016/j.ijhydene.2009.01.095
Acknowledgements
This work was financially supported by the National Natural Science Foundations of China (52261041) and the Natural Science Foundation of Inner Mongolia, China (2020LH01006, 2020LH05024 and 2022MS05011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, H., Yan, X., Li, X. et al. Microstructure characteristics and hydrogen storage kinetic of nano MgNi-REO alloys. Carbon Lett. 33, 2211–2222 (2023). https://doi.org/10.1007/s42823-023-00557-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00557-5