Abstract
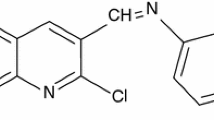
A new Schiff base, N-(5-bromo-2-hydroxybenzylidene) isonicotinohydrazide (NBHI), has been synthesized and studied as an inhibitor of X70 carbon steel (CS) corrosion in aggressive 1 M HCl environment using gravimetric, electrochemical impedance spectroscopy (EIS) tests, potentiodynamic polarization (PDP) and scanning electron microscopy (SEM). The inhibition efficiency was found to increase with increase in NBHI concentration but decreased with rise in temperature. Inhibition efficiencies up to 96.7%, 97.0% and 94.2% were obtained respectively for weight loss, EIS and polarization measurements at 1.00 mM and 303 K. Polarization studies indicate that NBHI essentially behaved as a mixed-type inhibitor controlling both anodic and cathodic reactions. EIS results indicated that charge transfer resistance (Rct) increased while double layer capacitance (Cdl) decreased with increasing NBHI concentration. The adsorption of NBHI onto the X70 CS surface obeyed Langmuir adsorption isotherm. SEM analysis supported the protective film formation of NBHI on the steel surface. Quantum chemical calculations were used to study NBHI reactivity and the results complimented well with the experimental data.







Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Abdallah M, Atwa ST, Salem MM, Fouda AS (2013) Synergistic effect of some halide ions on the inhibition of zinc corrosion in hydrochloric acid by tetrahydro carbazole derivatives compounds. Int J Electrochem Sci 8:10001–10021
Abdallah M, Fawzy A, Hawsawi H, Abdel Hameed RS, Al-Juaid SS (2020) Estimation of water-soluble polymers (Poloxamer and pectin) as corrosion inhibitors for carbon steel in acidic medium. Int J Electrochem Sci 15:8129–8144. https://doi.org/10.20964/2020.08.73
Ansari KR, Quraishi MA, Singh A (2014) Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 79:5–15. https://doi.org/10.1016/j.corsci.2013.10.009
Arivazhagan M, Subhasini VP (2012) Quantum chemical studies on structure of 2-amino-5-nitropyrimidine. Spectrochim Acta A 91:402–410. https://doi.org/10.1016/j.saa.2012.02.018
ASTM (2004) Standard practices for laboratory immersion corrosion testing of metals, American society for testing and materials G31-72. International, ASTM
Belfilali I, Chetouani A, Hammouti B, Aouniti A, Louhibi S, Al-Deyab SS (2012) Synthesis and application of 1,7−bis(2-HydroxyBenzamido)-4-Azaheptane and corrosion inhibitor of mild steel in molar hydrochloric acid medium. Int J Electrochem Sci 7:3997–4013
Bentrah H, Rahali Y, Chala A (2014) Gum Arabic as an eco-friendly inhibitor for API, 5L X42 pipeline steel in HCl medium. Corros Sci 82:426–431. https://doi.org/10.1016/j.corsci.2013.12.018
Boumhara K, Bentiss F, Tabyaoui M, Hammouti B (2014) Use of Artemisia Mesatlantica essential oil as green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. Int J Electrochem Sci 9:1187–1206
Chakravarthy MP, Mohana KN (2013) Inhibition behaviour of some isonicotinic acid hydrazides on the corrosion of mild steel in hydrochloric acid solution. Int J Corros:1–13. https://doi.org/10.1155/2013/854781
Dueke-Eze CU, Fasina TM, Oluwalana AE, Familoni OB, Mphalele JM, Onubuogu C (2020) Synthesis and biological evaluation of copper and cobalt complexes of (5-substituted-salicylidene) isonicotinichydrazide derivatives as antitubercular agents. Sci Afr 9:e00522. https://doi.org/10.1016/j.sciaf.2020.e00522
Ekanem UF, Umoren SA, Udousoro II, Udoh AP (2010) Inhibition of mild steel corrosion in HCl using pineapple leaves- (Ananas Comosus L.) extract. J Mater Sci 45:5558–5566
El Kacimi Y, Touir R, Galai M, Alaoui K, Dkhireche N, Touhami ME (2020) Relationship between silicon, phosphorus content and grain number in mild steels and its corrosion resistance in pickling hydrochloric acid. Int J Ind Chem 11:111–122. https://doi.org/10.1007/s40090-020-00206-0
Farag AA, Hegazy MA (2013) Synergistic inhibition effect of potassium iodide and novel Schiff bases on X65 steel corrosion in 0.5 M H2SO4. Corros Sci 74:168–177. https://doi.org/10.1016/j.corsci.2013.04.039
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41. https://doi.org/10.1016/j.corsci.2014.04.044
Hegazy MA, Abdallah M, Ahmed H (2010) Novel cationic gemini surfactants as corrosion inhibitors for carbon steel pipelines. Corros Sci 52(9):2897–2904. https://doi.org/10.1016/j.corsci.2010.04.034
Hegazy MA, Hasan AM, Emara MM, Bakr MF, Youssef AH (2012) Evaluating four synthesized Schiff bases as corrosion inhibitors on the carbon steel in 1 M hydrochloric acid. Corros Sci 65:67–76. https://doi.org/10.1016/j.corsci.2012.08.005
Herrag L, Hammouti B, Elkadiri S, Aouniti A, Jama C, Vezin H, Bentiss F (2010) Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: experimental and theoretical investigations. Corros Sci 52:3042–3051. https://doi.org/10.1016/j.corsci.2010.05.024
Iroha NB, Akaranta O (2020) Experimental and surface morphological study of corrosion inhibition of N80 carbon steel in HCl stimulated acidizing solution using gum exudate from Terminalia Mentaly. SN Appl Sci 2:1514. https://doi.org/10.1007/s42452-020-03296-8
Iroha NB, Dueke-Eze CU (2021) Experimental studies on two isonicotinohydrazide-based Schiff bases as new and efficient inhibitors for pipeline steel erosion corrosion in acidic cleaning solution. Chem Afr 4(3):635–646. https://doi.org/10.1007/s42250-021-00252-w
Iroha NB, Hamilton-Amachree A (2018) Adsorption and anticorrosion performance of Ocimum Canum extract on mild steel in sulphuric acid pickling environment. Am J Mater Sci 8(2):39–44. https://doi.org/10.5923/j.materials.20180802.03
Iroha NB, James AO (2018) Assessment of performance of velvet tamarind-furfural resin as corrosion inhibitor for mild steel in acidic solution. J Chem Soc Nigeria 43(3):510–517
Iroha NB, Nnanna LA (2021) Tranexamic acid as novel corrosion inhibitor for X60 steel in oil well acidizing fluids: surface morphology, gravimetric and electrochemical studies. Prog Color Colorants Coat 14:1–11
Iroha NB, Oguzie EE, Onuoha GN, Onuchukwu AI (2005) Inhibition of mild steel corrosion in acidic solution by derivatives of diphenyl glyoxal, 16th international corrosion congress. Bei**g, China
Iroha NB, Akaranta O, James AO (2015) Red onion skin extract-formaldehyde resin as corrosion inhibitor for mild steel in hydrochloric acid solution. Int Res J Pure App Chem 6(4):174–181. https://doi.org/10.9734/IRJPAC/2015/9555
Iroha NB, Madueke NA, Mkpenie V, Ogunyemi BT, Nnanna LB, Singh S, Akpan ED, Ebenso EE (2020) Experimental, adsorption, quantum chemical and molecular dynamics simulation studies on the corrosion inhibition performance of Vincamine on J55 steel in acidic medium. J Mol Struct 129533. https://doi.org/10.1016/j.molstruc.2020.129533
Iroha NB, Dueke-Eze CU, James AO, Fasina TM (2021) Newly synthesized N-(5-nitro-2-hydroxybenzylidene) pyridine-4-amine as a high-potential inhibitor for pipeline steel corrosion in hydrochloric acid medium. Egypt J Pet 30:55–61. https://doi.org/10.1016/j.ejpe.2021.02.003
Issaadi S, Douadi T, Zouaoui A, Chafaa S, Khan MA, Bouet G (2011) Novel thiophene symmetrical Schiff base compounds as corrosion inhibitor for mild steel in acidic media. Corros Sci 53:1484–1488. https://doi.org/10.1016/j.corsci.2011.01.022
James AO, Iroha NB (2019) An investigation on the inhibitory action of modified almond extract on the corrosion of Q235 mild steel in acid environment. IOSR J Appl Chem 12:1–10. https://doi.org/10.9790/5736-1202020110
James AO, Iroha NB (2021) New green inhibitor of Olax subscorpioidea root for J55 carbon steel corrosion in 15% HCl: theoretical, electrochemical, and surface morphological investigation. Emerg Mater. https://doi.org/10.1007/s42247-021-00161-1
Khaled KF, Elhabib OA, El-mghraby A, Ibrahim OB, Ibrahim MAM (2010) Inhibitive effect of thiosemicarbazone derivative on corrosion of mild steel in hydrochloric acid solution. J Mater Environ Sci 1:139–150
Khalil N (2003) Quantum chemical approach of corrosion inhibition. Electrochim Acta 48:2635–2640. https://doi.org/10.1016/S0013-4686(03)00307-4
Lebrini M, Traisnel M, Lagrenee M, Mernari B, Bentiss F (2008) Inhibitive properties, adsorption and a theoretical study of 3,5-bis( n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild steel in perchloric acid. Corros Sci 50:473–479. https://doi.org/10.1016/j.corsci.2007.05.031
Li X, Deng S, Fu H (2011) Triazolyl blue tetrazolium bromide as a novel corrosion inhibitor for steel in HCl and H2SO4 solutions. Corros Sci 53:302–309. https://doi.org/10.1016/j.corsci.2010.09.036
Lukovits I, Kálmán E, Zucchi F (2001) Corrosion inhibitors-correlation between electronic structure and efficiency. Corrosion 57:3–8
Madkour LH, Elroby SK (2015) Inhibitive properties, thermodynamic, kinetics and quantum chemical calculations of polydentate Schiff base compounds as corrosion inhibitors for iron in acidic and alkaline media. Int J Ind Chem 6(3):165–184. https://doi.org/10.1007/s40090-015-0039-7
Madueke NA, Iroha NB (2018) Protecting Aluminium alloy of type AA8011 from acid corrosion using extract from Allamanda cathartica leaves. Int J Innov Res Sci Eng Technol 7:10251–10258. https://doi.org/10.15680/IJIRSET.2018.0710014
Maduelosi NJ, Iroha NB (2021) Insight into the adsorption and inhibitive effect of spironolactone drug on C38 carbon steel corrosion in hydrochloric acid environment. Journal of Bio- and Tribo-Corrosion 7:6. https://doi.org/10.1007/s40735-020-00441-z
Mashuga ME, Olasunkanmi LO, Adekunle AS, Yesudass S, Kabanda MM, Ebenso EE (2015) Adsorption, thermodynamic and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as doi: corrosion inhibitors for mild steel in HCl. Materials 8:3607–3632. https://doi.org/10.3390/ma8063607
Messali M, Lgaz H, Dassanayake R, Salghi R, Jodeh S, Abidi N, Hamed O (2017) Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: electrochemical, Surface, DFT and MD Simulations Studies. J Mol Struct 1145:43–54. https://doi.org/10.1016/j.molstruc.2017.05.081
Mohan P, Usha R, Kalaignan GP, Muralidharan VS (2013) Inhibition effect of benzo hydrazide derivatives on corrosion behaviour of mild steel in 1 M HCl. J Chem:1–7
Murulana LC, Singh AK, Shukla SK, Kabanda MM, Ebenso EE (2012) Experimental and quantum chemical studies of some bis (trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind Eng Chem Res 51:13282–13299. https://doi.org/10.1021/ie300977d
Narang R, Narasimhan B, Sharma S (2012) A review on biological activities and chemical synthesis of hydrazide derivatives. Curr Med Chem 19:569–612. https://doi.org/10.2174/092986712798918789
Noor EA, Al-Moubaraki AH (2008) Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4-styryl pyridinium iodides/hydrochloric acid systems. Mater Chem Phys 110:145–154. https://doi.org/10.1016/j.matchemphys.2008.01.028
Okey NC, Obasi NL, Ejikeme PM, Ndinteh DT, Ramasami P, Sherif EM, Akpan ED, Ebenso EE (2020) Evaluation of some amino benzoic acid and 4-aminoantipyrine derived Schiff bases as corrosion inhibitors for mild steel in acidic medium: synthesis, experimental and computational studies. J Mol Liq 315:113773. https://doi.org/10.1016/j.molliq.2020.113773
Ramaganthan B, Gopiraman M, Olasunkanmi LO, Kabanda MM, Yesudass S, Bahadur I, Adekunle AS, Obot IB, Ebenso EE (2015) Synthesized photo-cross-linking chalcones as novel corrosion inhibitors for mild steel in acidic medium: experimental, quantum chemical and Monte Carlo simulation studies. RSC Adv 5:76675–76688. https://doi.org/10.1039/C5RA12097G
Sainia M, Kumar P, Kumar M, Kalavathy R, Mani V, Mishra RK, Majeed ABA, Narasimhana B (2014) Synthesis, in vitro antimicrobial, anticancer evaluation and QSAR studies of N’-(substituted)-4-(butan-2-lideneamino)benzo hydrazides. Arab J Chem 7:448–460. https://doi.org/10.1016/j.arabjc.2013.05.010
Singh A, Lin Y, Obot IB, Ebenso EE, Ansari KR, Quraishi MA (2015) Corrosion mitigation of J55 steel in 3.5% NaCl solution by a macrocyclic inhibitor. Appl Surf Sci 356:341–347. https://doi.org/10.1016/j.apsusc.2015.08.094
Umoren SA (2016) Biomaterials for corrosion protection: evaluation of mustard seed extract as eco-friendly corrosion inhibitor for X60 steel in acid media. J Adhesion Sci Technol 30:1858–1879. https://doi.org/10.1080/01694243.2016.1168339
Yadav M, Kumar S, Sinha RR, Behera D (2013) Experimental and quantum chemical studies on corrosion inhibition performance of Benzimidazole derivatives for mild steel in HCl. Ind Eng Chem Res 52:6318–6328. https://doi.org/10.1021/ie400099q
Zeino A, Abdulazeez I, Khaled M, Jawich MW, Obot IB (2018) Mechanistic study of polyaspartic acid (PASP) as eco-friendly corrosion inhibitor on mild steel in 3% NaCl aerated solution. J Mol Liq 250:50–62. https://doi.org/10.1016/j.molliq.2017.11.160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chokor, A.A., Dueke-Eze, C.U., Nnanna, L.A. et al. Adsorption, electrochemical and theoretical studies on the protective effect of N-(5-bromo-2-hydroxybenzylidene) isonicotinohydrazide on carbon steel corrosion in aggressive acid environment. Saf. Extreme Environ. 4, 35–45 (2022). https://doi.org/10.1007/s42797-022-00050-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42797-022-00050-8