Abstract
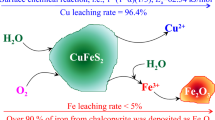
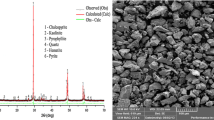
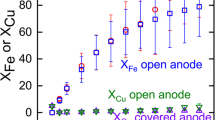
The formation of a passive layer during the hydrometallurgical leaching of copper from chalcopyrite leads to a relatively low copper recovery owing to the halting of the leaching efficiency. Thus, the copper recovery during chalcopyrite leaching needs to be promoted and the formation of passive layers on the mineral surface must be reduced. Organic solvents and surfactants are used to thin the passive layer, improve electron transfer through the passive layer using electrical conductors, and improve leaching through the galvanic effect. Recent research that combined the galvanic effect and enhancement of the electrical conductivity of passive layers has been intensively discussed. Therefore, in this study, we determine the effect of carbon black on the leaching mechanism of chalcopyrite in the presence of manganese dioxide and pyrite. Chalcopyrite leaching is performed using a 1 M sulfuric acid solution under ambient conditions. Inductively coupled plasma-optical emission spectrometry is used to measure the mass concentrations of the copper and iron ions, and an X-ray diffraction pattern of the solid residue after leaching is obtained. Moreover, a cyclic voltammogram is obtained by measuring the current at the working electrode, which is prepared using pure chalcopyrite and pyrite, to reveal the role of carbon black in the leaching process. The copper recovery of our CuFeS2 + FeS2 + MnO2 + C system (91.6%) is significantly higher than that of a CuFeS2 + FeS2 system (1.0%) after 1 M sulfuric acid leaching owing to the galvanic effect. In addition, the results indicate that the electron transfer reaction associated with chalcopyrite, chalcocite, pyrite, and elemental sulfur occurs, which leads to the continuous leaching of chalcopyrite into copper minerals, including chalcopyrite and covellite. When chalcopyrite is leached in sulfuric acid with manganese dioxide, the passive layers are activated by the influence of carbon black. Carbon black served as the active site for the reaction, enhancing the effect of charge carriers and facilitating additional oxidation half-reactions. This ultimately led to improved chalcopyrite leaching and a continuous increase in copper recovery.
Graphical Abstract







Similar content being viewed by others
References
An Z, Yan J, An H et al (2023) Resource, environmental and economics research for primary and secondary copper: a bibliometric and systematic review. J Clean Prod 425:138671. https://doi.org/10.1016/j.jclepro.2023.138671
Kang X, Wang M, Chen L, Li X (2023) Supply risk propagation of global copper industry chain based on multi-layer complex network. Resour Policy 85:103797. https://doi.org/10.1016/j.resourpol.2023.103797
Schlesinger ME (2011) Extractive metallurgy of copper, 5th edn. Elsevier, Oxford
Bogdanović GD, Petrović S, Sokić M, Antonijević MM (2020) Chalcopyrite leaching in acid media. Metall Mater Eng 26:177–198. https://doi.org/10.30544/526
Zhao H, Yang C, Zhang X et al (2021) Biohydrometallurgy of chalcopyrite. Elsevier, Amsterdam
O’Connor GM, Eksteen JJ (2020) A critical review of the passivation and semiconductor mechanisms of chalcopyrite leaching. Miner Eng 154:106401. https://doi.org/10.1016/j.mineng.2020.106401
Watling HR (2013) Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 140:163–180. https://doi.org/10.1016/j.hydromet.2013.09.013
Solis-Marcíal OJ, Lapidus GT (2013) Improvement of chalcopyrite dissolution in acid media using polar organic solvents. Hydrometallurgy 131–132:120–126. https://doi.org/10.1016/j.hydromet.2012.11.006
Devi NB, Madhuchhanda M, Rao KS et al (2000) Oxidation of chalcopyrite in the presence of manganese dioxide in hydrochloric acid medium. Hydrometallurgy 57:57–76. https://doi.org/10.1016/S0304-386X(00)00100-6
Gantayat BP, Rath PC, Paramguru RK, Rao SB (2000) Galvanic interaction between chalcopyrite and manganese dioxide in sulfuric acid medium. Metall and Mater Trans B 56:55–61. https://doi.org/10.1007/s11663-000-0130-z
Koleini SMJ, Aghazadeh V, Sandström Å (2011) Acidic sulphate leaching of chalcopyrite concentrates in presence of pyrite. Miner Eng 24:381–386. https://doi.org/10.1016/j.mineng.2010.11.008
Martínez-Gómez VJ, Fuentes-Aceituno JC, Pérez-Garibay R, Lee JC (2018) A study of the electro-assisted reductive leaching of a chalcopyrite concentrate in HCl solutions. Part I: Kinetic behavior and nature of the chalcopyrite reduction. Hydrometallurgy 181:195–205. https://doi.org/10.1016/j.hydromet.2018.09.012
Prasad S, Pandey BD (1998) Alternative processes for treatment of chalcopyrite. Miner Eng 11:763–781. https://doi.org/10.1016/S0892-6875(98)00061-2
Nazari G, Dixon DG, Dreisinger DB (2012) The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the Galvanox™ process. Hydrometallurgy 113–114:122–130. https://doi.org/10.1016/j.hydromet.2011.12.011
Nakazawa H, Nakamura S, Odashima S, Hareyama W (2016) Effect of carbon black to facilitate galvanic leaching of copper from chalcopyrite in the presence of manganese (IV) oxide. Hydrometallurgy 163:69–76. https://doi.org/10.1016/j.hydromet.2016.03.003
Antonijević MM, Bogdanović GD (2004) Investigation of the leaching of chalcopyritic ore in acidic solutions. Hydrometallurgy 73:245–256. https://doi.org/10.1016/j.hydromet.2003.11.003
Hackl RP, Dreisinger DB, Peters E, King JA (1995) Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 39:25–48. https://doi.org/10.1016/0304-386X(95)00023-A
Lu ZY, Jeffrey MI, Lawson F (2000) An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 56:145–155. https://doi.org/10.1016/S0304-386X(00)00068-2
Eghbalnia M, Dixon DG (2011) Electrochemical study of leached chalcopyrite using solid paraffin-based carbon paste electrodes. Hydrometallurgy 110:1–12. https://doi.org/10.1016/j.hydromet.2011.07.009
Toro N, Pérez K, Saldaña M et al (2020) Dissolution of pure chalcopyrite with manganese nodules and waste water. J Market Res 9:798–805. https://doi.org/10.1016/j.jmrt.2019.11.020
Parker AJ, Paul RL, Power CP (1981) Electrochemistry of the oxidative leaching of copper from chalcopyrite. J Electroanal Chem 118:305–316. https://doi.org/10.1016/S0022-0728(81)80549-9
Ghomi MA, Mozammel M, Moghanni H, Shahkar L (2019) Atmospheric leaching of chalcopyrite in the presence of some polar organic reagents: a comparative study and optimization. Hydrometallurgy 189:105120. https://doi.org/10.1016/j.hydromet.2019.105120
Castillo-Magallanes N, Cruz R, Lázaro I (2020) Effect of organic agents on the oxidation process of chalcopyrite in a sulfuric acid solution. Electrochim Acta 355:136789. https://doi.org/10.1016/j.electacta.2020.136789
Nakazawa H, Yamamoto H (2017) Effect of carbon black on copper ore leaching in sulfuric acid media at 50°C. Resour Process 64:90–98. https://doi.org/10.4144/rpsj.64.90
Tanne C, Schippers A (2021) Electrochemical investigation of microbially and galvanically leached chalcopyrite. Hydrometallurgy 202:105603. https://doi.org/10.1016/j.hydromet.2021.105603
Petrovic SJ, Bogdanovic GD, Antonijevic MM (2018) Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans Nonferrous Metals Soc China (English Edition) 28:1444–1455. https://doi.org/10.1016/S1003-6326(18)64788-0
Córdoba EM, Muñoz JA, Blázquez ML et al (2009) Comparative kinetic study of the silver-catalyzed chalcopyrite leaching at 35 and 68°C. Int J Miner Process 92:137–143. https://doi.org/10.1016/j.minpro.2009.03.007
Ganbari AA, Erfan AF, Arvin TT, Hossein A (2020) Comparison of the effect of NaClO3 and H2O2 on the molybdenum leaching from molybdenite concentrate. Trans Indian Inst Met 73:2355–2360. https://doi.org/10.1007/s12666-020-02036-1
Shoghian-Alanaghi A, Zamharir AJ, Aghajani H, Arvin TT (2022) Improving the leaching rate of molybdenite concentrate using oxidants by adding ethylene glycol and oxygen: kinetic study. Min Metall Explor 39:1753–1761. https://doi.org/10.1007/s42461-022-00642-9
Hong M, Huang X, Gan X et al (2021) The use of pyrite to control redox potential to enhance chalcopyrite bioleaching in the presence of Leptospirillum ferriphilum. Miner Eng 172:107145. https://doi.org/10.1016/j.mineng.2021.107145
Atkins P, de Paula J (2014) Physical chemistry, 10th edn. Oxford University Press, New York
Abed N (1999) A fundamental study of the reductive leaching of chalcopyrite using metallic iron. The University of British Columbia, Master degree
Chang KX, Zhang YS, Zhang JM et al (2019) Effect of temperature-induced phase transitions on bioleaching of chalcopyrite. Trans Nonferrous Met Soc China (English Edition) 29:2183–2191. https://doi.org/10.1016/S1003-6326(19)65124-1
Arce EM, González I (2002) A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulfuric acid solution. Int J Miner Process 67:17–28. https://doi.org/10.1016/S0301-7516(02)00003-0
Asgari K, Hassanzadeh A, Nazari S et al (2021) Effect of externally adding pyrite and electrical current on galvanic leaching of chalcopyrite concentrate. Physicochem Probl Miner Process 57:105–119. https://doi.org/10.37190/PPMP/132956
Chung DDL (2004) Electrical applications of carbon materials. J Mater Sci 39:2645–2661. https://doi.org/10.1023/B:JMSC.0000021439.18202.ea
Huai Y, Plackowski C, Peng Y (2018) The galvanic interaction between gold and pyrite in the presence of ferric ions. Miner Eng 119:236–243. https://doi.org/10.1016/j.mineng.2018.01.040
Ahmadi A, Ranjbar M, Schaffie M (2012) Catalytic effect of pyrite on the leaching of chalcopyrite concentrates in chemical, biological and electrobiochemical systems. Miner Eng 34:11–18. https://doi.org/10.1016/j.mineng.2012.03.022
Hong M, Wang X, Wu L et al (2019) Intermediates transformation of bornite bioleaching by Leptospirillum ferriphilum and Acidithiobacillus caldus. Minerals 9:1–14. https://doi.org/10.3390/min9030159
Sequoia E, Holliday RI, Richmond WR (1990) An electrochemical study of the oxidation of chalcopyrite in acidic solution. J Electroanal Chem 288:83–98. https://doi.org/10.1016/0022-0728(90)80027-4
Liu Y, Wu S, Jia S et al (2019) Oxidation characteristics of the reactivity between pyrite and aqueous arsenate. Trans Tian** Univ 25:371–380. https://doi.org/10.1007/s12209-019-00190-2
Funding
This work was supported by the Fellow research grant of National University of Mongolia (P2022-4394).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amarsanaa, A., Damdin, NE., Nyamdorj, B. et al. Mechanism of Enhanced Copper Recovery From Chalcopyrite in the Presence of Carbon Black Under Ambient Conditions. Mining, Metallurgy & Exploration (2024). https://doi.org/10.1007/s42461-024-01014-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42461-024-01014-1