Abstract
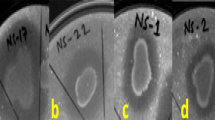
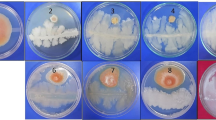
In the present study, twenty-six rhizobacterial antagonists of Fusarium oxysporum f. sp. ciceris (Foc), isolated from Foc infected chickpea fields were evaluated for their protein secretion. Exoprotein liberation by rhizobacterial antagonists lied in the range between 28.2 and 795 μg/ml, where maximum exoprotein was recorded by isolate Ps47, followed by Ps45 (795.0 µg/ml) and Ps44 (578.2 μg/ml). Further observation of hydrolytic proteins, revealed most of the bacterial antagonists to exhibit proteolytic, amylolytic, chitinolytic as well as cellulose degrading potential on minimal agar plates, supplemented with respective substrates. About 73 and 80% of the antagonists were found positive for chitinolytic and cellulolytic activity respectively, where isolate Ba18 scored maximum hydrolytic index for chitin and Ba10 exhibited highest cellulose hydrolytic index of 2.1. Assay for proteases, denoted 84.6% of isolates to produce lysis zones, when gelatin was used as protein source, where 80% of isolates showed hydrolytic activity for casein supplemented minimal salt (MS) agar. SDS-PAGE analysis for the presence of hydrolytic enzymes in the extracellular crude protein revealed Pseudomonas isolate Ps45 and Bacillus Ba1a to exhibit a range of protein bands between 5 and 150 kDa, belonging to several group of proteases, amylases, chitinases and cellulases. Scanning Electron Microscopic examinations of the interaction zones between fungal pathogen (Foc) and isolate Ps45, revealed hyphal distortion due to lysis effect with scanty mycelial growth compared to control, that was maintained without any bacterial treatment. Screening and characterization of bacterial strains with the ability to produce a range of lytic enzymes will greatly enhance our understanding for their exploitation in the management of soil borne fungal pathogens.





Similar content being viewed by others
References
Abdel-Monaim MF, Mahmoud E, Ibrahim M, Fatma AM, Zeinab NH, Soliman S, Sobeiha A, Fatma M, Waked DA, Eleawa M, Salama A (2016) Efficacy of secondary metabolites and extracellular lytic enzymes of plant growth promoting rhizobacteria (PGPR) in controlling fusarium wilt of chickpea. Egypt J Agric Res 94(3):573–589
Ahemad M, Kibret M (2014) Mechanisms and application s of plant growth promoting rhizobacteri a: current perspective. J King Saud Univ Sci 26:1–20
Ajit NS, Verma R, Shanmugam V (2006) Extracellular chitinases of fluorescent pseudomonads antifungal to Fusarium oxysporum f. sp. dianthi causing carnation wilt. Curr Microbiol 52(4):310–316
Akbalık G (2003) Screening for industrially important extracellular enzymes from alkalophilic Bacillus genus, Master’s thesis, İzmir Institute of Technology.
Annamalai N, Rajeswari MV, Balasubramanian T (2014) Extraction, purification and application of thermostable and halostable alkaline protease from Bacillus alveayuensis CAS 5 using marine waste. Food Bioprod Process 92:335–342
Ardura A, Ana LR, Vazquez EG (2013) Genetic detection of Pseudomonas spp. in commercial amazonian fish. Int J Environ Res Public Health 10:3954–3966
Avinash TS, Rai VR (2016) Plant growth promoting rhizobacteria: a boon to agriculture. Int J Agric Food Sci 6(2):28–36
Aziz LM, Hamza SJ, Rahman AA (2012) Isolation and characterization of phenazine produced from mutant Pseudomonas aeruginosa. Al Anbar J Vet Sci 5(1):42–52
Bach ED, Santos SGD, de Carvalho FG, Lisboa BB, Passaglia LMP (2016) Evaluation of biological control and rhizosphere competence of plant growth promoting bacteria. Appl Soil Ecol 99:141–149
Basha S, Ulaganathan K (2002) Antagonism of Bacillus species (strain BC121) towards Curvularia lunata. Curr Sci 25:1457–1463
Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M (2014) Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68(1):71–81
Chaiharn M, Chunhaleuchanon S, Kozo A, Lumyong S (2008) Screening of rhizobacteria for their plant growth promoting activities. Curr Appl Sci Technol 8(1):18–23
Chang WT, Wag CM (2010) An antifungal chitinase produced by Bacillus subtilis using chitin waste as a crabon source. World J Microbiol Biotechnol 26:945–950
Chao-Lin L, Chia-Rui S, Fong-Fu H, Jeen-Kuan C, Pei-Tzu W, Shang- Hsin G, Wen-Chien L, Feng-Wei Y, Zachary BM, John T, Grosx ML (2009) Isolation and identification of two novel SDS-resistant secreted chitinases from Aeromonas schubertii. Biotechnol Prog 25(1):124–131
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Dunne C, Moe-Loccoz Y, Bruijn FJ, O’Gara F (2000) Overproduction of an inducible extracellular serine protease improves biological control of Pythium ultimum by Stenotrophomonas maltophilia strain W81. Microbiology 146:2069–2078
Fernando WGD, Nakkeeran S, Zhang Y (2006) Biosynthesis of antibiotics by PGPR and its relation in biocontrol of plant diseases. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Netherlands, pp 67–109
Fira D, Dimkić I, Berić T, Lozo J, Stanković S (2018) Biological control of plant pathogens by Bacillus species. J Biotechnol 285:44–55
Gajera HP, Savaliya DD, Hirapara DG, Patel SV, Golakiya BA (2016) Biocontrol mechanism of bacillus for fusarium wilt management in cumin (Cuminum cyminum L.). Current trends in plant disease diagnostics and management practices. Springer, Cham, pp 29–47
Gomez-Ramırez M, Rojas-Avelizapa LI, Rojas-Avelizapa NG, Cruz-Camarillo RC (2004) Colloidal chitin stained with Remazol Brilliant Blue R, a useful substrate to select chitinolytic microorganisms and to evaluate chitinases. J Microbiol Methods 56:213–219
Gopalakrishnan S, Vadlamudi S, Samineni S, Kumar CVS (2016) Plant growth-promotion and biofortification of chickpea and pigeonpea through inoculation of biocontrol potential bacteria, isolated from organic soils. Springer plus 5:1882–1893
Granjo CA, Dos RTA, Gambale W, Corrêa B (2007) Morphogenesis and growth kinetics of Fusarium verticillioides. Mycopathologia 164(3):119–126
Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7(2):96–102
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion. A review. Ann Microbiol 60(4):5795–5798
Huang LQ, Niu YC, Su L, Deng H, Lyu H (2020) The potential of endophytic fungi isolated from cucurbit plants for biocontrol of soilborne fungal diseases of cucumber. Microbiol Res 231:126369
Jadhav HP, Shaikh SS, Sayyed RZ (2017) Role of hydrolytic enzymes of rhizoflora in biocontrol of fungal phytopathogens: an overview. Rhizotrophs: plant growth promotion to bioremediation. Springer, Singapore, pp 183–203
Jensen DF, Karlsson M, Sarrocco S, Vannacci G (2016) Biological control using microorganisms as an alternative to disease resistance. Plant Pathogen Resistance Biotechnol 341–363
Joshi M, Shrivastava R, Sharma AK, Prakash A (2012) Screening of resistant verities and antagonistic Fusarium oxysporum for biocontrol of Fusarium Wilt of Chilli. Plant Pathol Microbiol 3(134):2
Kai M, Effmert U, Berg G, Piechulla B (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol 187:351–360
Kamensky M, Ovadis M, Chet I, Chernin L (2003) Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biol Biochem 35:323–331
Kim YC, Glick B, Bashan Y, Ryu CM (2013) Enhancement of plant drought tolerance by microbes. In: Aroca R (ed) Plant responses to drought stress. Springer, Berlin, pp 45–51
Kohring S, Wiegel J, Mayer F (1990) Subunit composition and glycosidic activities of the cellulase complex from Clostridium thermocellum JW20. Appl Environ Microbiol 56(12):3798–3804
Kumar PA, Johri BN (2011) Bacillus as PGPR in crop ecosystem. In: Maheshwari DK (ed) Bacteria in agrobiology: crop ecosystems. Springer, Berlin, pp 37–59
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167(8):493–499
Kumari S, Khanna V (2014) Effect of antagonistic rhizobacteria coinoculated with Mesorhizobium ciceris on control of fusarium wilt in chickpea (Cicer arietinum L.). Afric J Micro Res 8(12):1255–1265
Kumari S, Khanna V (2018) Biological management of vascular wilt of chickpea (Cicer arietinum L.) incited by Fusarium oxysporum f. sp. ciceris by antagonistic rhizobacteria co-inoculated with native mesorhizobium. Int J Curr Microbiol App Sci 7(1):920–941
Kumari S, Khanna V (2019) Biocidal mechanisms in biological control of fusarium wilt in chickpea (Cicer arietinum L.) by antagonistic rhizobacteria: a current perspective in soil borne fungal pest management. Int J Curr Microbiol App Sci 8(10):1494–1510
Kumari S, Khanna V (2020) Induction of systemic resistance in chickpea (Cicer arietinum L.) against Fusarium oxysporum f. sp. ciceris by antagonistic rhizobacteria in assistance with native mesorhizobium. Curr Microbiol 77(1):85–98
Kumari P, Khanna V, Sharma P, Kumari S (2016) Allelopathic effects of native Bacillus sp. against Fusarium oxysporum causing chickpea wilt. Allelopath J 38:77–90
Kumudini BS, Jayamohan NS, Patil SV (2017) Integrated mechanisms of plant disease containment by rhizospheric bacteria: Unraveling the signal cross talk between plant and fluorescent Pseudomonas. In: Agriculturally important microbes for sustainable agriculture. Springer, Singapore, pp. 263–291
Leon M, Yaryura PM, Montecchia MS, Hernandez AI, Correa OS, Pucheu NL, Kerber NL, Garcia AF (2009) Antifungal activity of selected indigenous Pseudomonas and Bacillus from the soybean rhizosphere. Int J Microbiol 5:1–9
Lowry OH, Rosbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin Phenol reagent. J Biol Chem 193:265–370
Maharana AK, Ray P (2013) Isolation and screening of cold active extracellular enzymes producing psychrotrophic bacteria from soil of Jammu City. Biosci Biotechnol Res Asia 10(1):267–273
Maksimov I, Abizgildina RR, Pusenkova LI (2011) Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens. Appl Biochem Microbiol 47:333–345
Mala M, Srividya S (2010) Partial purification and properties of a laundry detergent compatible alkaline protease from a newly isolated Bacillus species Y. Indian J Microbiol 50:309–317
Manjula K, Poldile AR (2005) Production of fungal cell wall degrading enzymes by biocontrol strain of Bacillus subtilis A F 1. Indian J Exp Biol 43:892–896
Nadeem SM, Naveed M, Zahir ZA, Asghar HN (2013) Plant–microbe interactions. Plant microbe symbiosis: fundamentals and advances. Springer, India, pp 51–103
Namasivayam E, Ravindar JD, Mariappan K, Akhil J, Mukesh K, Jayara R (2011) Production of extracellular pectinase by Bacillus cereus isolated from market solid waste. J Bioanal Biomed 3(3):70–75
Neeraja C, Anil K, Purushotham P, Suma K, Sarma P, Moerschbacher BM, Podile AR (2010) Biotechnological approaches to develop bacterial chitinases as a bioshield against fungal diseases of plants. Crit Rev Biotechnol 30:231–241
Nion YA, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. p ME14144
Pande S, Desai S, Sharma M (2010) Impacts of climate change on rainfed crop diseases: Current Status and Future Research Needs. Nat Symp Clim Change Rainfed Agric Hyderabad 18(20):55–59
Pushpavathi Y, Dash SN, Madhavi N, Deepika D (2016) Biological control of Fusarium wilt disease in banana with emphasis on Trichoderma spp. and Pseudomonas spp. Plant Arch 16(1):51–59
Radjacommare R, Venkatesan S, Samiyappan R (2010) Biological control of phytopathogenic fungi of vanilla through lytic action of Trichoderma species and Pseudomonas fluorescens. Arch Phytopathol Pflanzenschutz 43(1):1–17
Rajput RS, Singh P, Singh J, Ray S, Vaishnav A, Singh HB (2019) Seed biopriming through beneficial rhizobacteria for mitigating soil-borne and seed-borne diseases. In: Plant growth promoting rhizobacteria for sustainable stress management. Springer, Singapore. pp. 201–215
Ramesh R, Mathew T, Singh N (2009) Role of chitinolytic enzymes and volatile compounds produced by endophytic bacteria in the inhibition of mango (Mangifera indica L.) root rot pathogens. J Biol Control 23(4):433–441
Raza W, Ling N, Zhang R, Huang Q, Xu Y, Shen Q (2017) Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit Rev Biotechnol 37(2):202–212
Rezzonico F, Zala M, Keel C, Duffy B, Moénne-Loccoz Y, Défago G (2007) Is the ability of biocontrol fluorescent pseudomonads to produce the antifungal metabolite 2, 4-diacetylphloroglucinol really synonymous with higher plant protection. New Phytol 173:861–872
Ruiz-Sanchez A, Cruz-Camarillo R, Salcedo-Hernandez R, Barboza-Corona JE (2005) Chitinases from Serratia marcescens Nima. Biotechnol Lett 27:649–653
Sabaratnam S, Traquair JA (2015) Mechanism of antagonism by Streptomyces griseocarneus (strain Di944) against fungal pathogens of greenhouse-grown tomato transplants. Can J Plant Pathol 37(2):197–211
Sadfi N, Cherif M, Hajlaoui MR, Boudabbous A, Belanger R (2002) Isolation and partial purification of antifungal metabolites produced by Bacillus cereus. Ann Microbiol 52(3):323–338
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169(1):18–29
Sarian FD, van der Kaaij RM, Kralj S, Wijbenga DJ, Binnema DJ, Vander MMJ, Dijkhuizen L (2012) Enzymatic degradation of granular potato starch by Microbacterium aurum strain B8A. Appl Microbiol Biotechnol 93(2):645–654
Sasirekha B, Bedashree T, Champa KL (2012) Optimization and partial purification of extracellular phytase from Pseudomonas aeruginosa p6. Eur J Exp Biol 2(1):95–104
Schallmey M, Singh A, Ward OP (2004) Development in the use of Bacillus sp. for industrial production. Cna J Microbiol 50:1–9
Sekhon A, Dahiya N, Tewari RP, Hoondal GS (2006) Production of extracellular lipase by Bacillus megaterium AKG-1 in submerged fermentation. Indian J Biotechnol 5:179–183
Shahbazi M, Reza H, Heidari K (2012) A novel low molecular weight extracellular protease from a moderately halophilic bacterium Salinivibrio sp. strain MS-7: production and biochemical properties. Mol Biol Res Comm 1(2):45–56
Shalini SR (2007) Screening for antifungal activity of pseudomonas fluorescens against phytopathogenic fungi. Internet J Microbiol 5(2):1–6
Silva AKS, Silva TRN, Nicoli JR, Vasquez-Pinto LMC, Martins FS (2018) In vitro evaluation of antagonism, modulation of cytokines and extracellular matrix proteins by Bifidobacterium strains. Lett Appl Microbiol 67(5):497–505
Sindhu SS, Dadarwal KR (2001) Chitinolytic and cellulolytic Pseudomonas sp. antagonistic to fungal pathogens enhances nodulation by Mesorhizobium sp. cicer in chickpea. Microbiol Res 156(4):353–358
Singh A, Jain A, Sarma BK, Upadhyay RS, Singh HB (2014) Rhizosphere competent microbial consortium mediates rapid changesin phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol Res 169:353–360
Sku** JJ, Potgieter HJ, Alexunder M (1965) Dissolution of fungal cell wall by Streptomyces Chitinase and β(1–3) glucanase. Arch Biochem Biophy 111:358–364
Smolińska U, Kowalska B (2018) Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum––a review. J Pl Pathol 100(1):1–12
Solanki MK, Kumar S, Pandey AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol Sci Tech 22(2):203–217
Spilker T, Coenye T, Vandamme P, LiPuma JJ (2004) PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol 42:2074–2079
Sutrisno A, Ueda M, Abe Y, Nakazawa M, Miyatake KA (2004) Chitinase with high activity toward partially N-acetylated chitosan from a new, moderately thermophilic, chitin-degrading bacterium, Ralstonia sp. A-471. Appl Microbiol Biotechnol 63:398–406
Trotel-Aziz P, Couder M, Biagianti S, Aziz A (2008) Characterisation of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas sp. mediating grapevine resistance against Botrytis cinerea. Environ Exp Bot 64:21–32
Ueda M, Kotani Y, Sutrisno A, Nakazawa M, Miyatake K (2005) Purification and characterization of chitinase b from moderately thermophilic bacterium Ralstonia sp. A-471. Biosci Biotechnol Biochem 69(4):842–844
Upadyay SK, Maurya SK, Singh DP (2012) Salinity tolerance in free living plant growth promoting Rhizobacteria. Ind J Sci Res 3:73–78
Wang N, Liu M, Guo L, Yang X, Qiu D (2016) A novel protein elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 induces systemic resistance in tobacco. Int J Biol Sci 12(6):757–767
**an L, Wang F, Luo X, Feng YL, Feng JX (2015) Purification and characterization of a highly efficient calcium-independent α-amylase from Talaromyces pinophilus. PLoS ONE 10(3):1–95
Acknowledgements
The present investigation was conducted at Pulses section, Department of Plant Breeding and Genetics, Punjab Agricultural University. The authors are thankful to Department of Microbiology, Punjab Agricultural University, Ludhiana, Punjab, India for further support and Department of Science and Technology (DST), New Delhi, for funding as doctoral fellowship to the first as well as the corresponding author of the manuscript.
Funding
The authors are thankful to Punjab Agricultural University, Ludhiana, Punjab, India for providing facilities and Department of Science and Technology (DST), New Delhi, for funding as doctoral fellowship to the first as well as the corresponding author of the manuscript.
Author information
Authors and Affiliations
Contributions
First author: Dr. (Mrs.) SK (Corresponding Author). Specific contribution: Planning and execution of the represented research work, Writing and editing of the manuscript. Second author: Dr. (Mrs.) VK. Specific contribution: Planning of the experiments included in this study, reviewing the manuscript. Third author: Dr. AS. Specific contribution: Assistance in the experiments especially SDS-PAGE analysis included in this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The first author Dr. Suman Kumari declares that she has no conflict of interest. The second author Dr. Veena Khanna declare that she has no conflict of interest and the third author Dr. Alla Singh also declare that he has no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All the persons that have contributed to this manuscript are presented as authors in series according to his her/ contribution. There is no other person that has contributed and is not acknowledged. All the authors agree for their place in author list as presented/submitted.
Consent for publication
The corresponding author declare that no content/data or material has been presented in the manuscript other than the corresponding author’s of only this manuscript (with reference). All the authors declare the same.
Availability of data and material
The authors declare that the data supporting the findings of this study are available within the article itself.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, S., Khanna, V. & Singh, A. Characterization and evaluation of extracellular hydrolytic proteins from rhizobacterial antagonists isolated from Fusarium oxysporum f. sp. ciceris infected chickpea fields. Indian Phytopathology 75, 165–177 (2022). https://doi.org/10.1007/s42360-021-00443-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-021-00443-y