Abstract
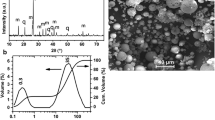
This study aims to evaluate the influence of a novel hydrothermal parameter, liquor/(Si/Al) ratio, on the crystallization of zeolite from fumed silica and fly ash by applying the Taguchi methodology. Template concentration, duration treatment, and crystallization temperature were adjusted to improve zeolitization yield. The resulting products were analyzed by XRD, FTIR, SEM–EDX, Raman microscopy, and nitrogen desorption–desorption experiments. The interaction of liquor/(Si/Al) parameters with considered factors was investigated by using ANOM and ANOVA analysis. The zeolitization content was considered as the measured response. The result indicated that a lower L/(Si/Al) ratio, with a contribution process of 20.42%, promoted a high zeolitization yield, while a higher L/(Si/Al) ratio involved a low zeolitization rate of starting materials. The medium temperature (120 °C), high NaOH concentration (2 M), medium treatment period (24 h), and lower L/(Si/Al) ratio (40) were shown to be the optimal conditions for maximum zeolitization yield. The zeolitization yield of started reactants reached 92.84% and Na-P1, Na-X, Na-Y, and analcime zeolites were the neoformed phases with a medium granulite area of 0.730 µm2 and surface area of 64.55 m2/g. Thus, the results demonstrate that carefully selecting operational factors may significantly influence zeolite synthesis.







Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included in the article.
References
Ait Baha A, Tabit K, Idouhli R et al (2023) Zeolitization of fumed silica and coal fly ash using the Taguchi method toward organic pollutant removal. SILICON 2023:1–12. https://doi.org/10.1007/S12633-023-02501-8
Sathupunya M, Gulari E, Wongkasemjit S (2003) Na-A (LTA) zeolite synthesis directly from alumatrane and silatrane by sol-gel microwave techniques. J Eur Ceram Soc 23:1293–1303. https://doi.org/10.1016/S0955-2219(02)00287-X
Wu Y, Ren X, Wang J (2009) Facile synthesis and morphology control of zeolite MCM-22 via a two-step sol–gel route with tetraethyl orthosilicate as silica source. Mater Chem Phys 113:773–779. https://doi.org/10.1016/J.MATCHEMPHYS.2008.08.008
Abdullahi T, Harun Z, Othman MHD (2017) A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv Powder Technol 28:1827–1840. https://doi.org/10.1016/J.APT.2017.04.028
Purnomo CW, Salim C, Hinode H (2012) Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous Mesoporous Mater 162:6–13. https://doi.org/10.1016/J.MICROMESO.2012.06.007
Mustakim SM, Dashhh SK, Mishra J et al (2020) Improvement in fresh mechanical and microstructural improvement in fresh, mechanical and microstructural properties of fly ash- blast furnace slag based geopolymer concrete by addition of nano and micro silica. SILICON. https://doi.org/10.1007/s12633-020-00593-0
Jithendra C, Elavenil S (2020) Effects of silica fume on workability and compressive strength properties of aluminosilicate based flowable geopolymer mortar under ambient curing. SILICON 12:1965–1974. https://doi.org/10.1007/s12633-019-00308-0
Calabrese L, Bonaccorsi L, Caprì A, Proverbio E (2014) Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog Org Coat 77:1341–1350. https://doi.org/10.1016/J.PORGCOAT.2014.04.025
Jan A, Pu Z, Khan KA et al (2022) A review on the effect of silica to alumina ratio, alkaline solution to binder ratio, calcium oxide + ferric oxide, molar concentration of sodium hydroxide and sodium silicate to sodium hydroxide ratio on the compressive strength of geopolymer concrete. SILICON 14:3147–3162. https://doi.org/10.1007/s12633-021-01130-3
Blissett RS, Rowson NA (2012) A review of the multi-component utilisation of coal fly ash. Fuel 97:1–23. https://doi.org/10.1016/J.FUEL.2012.03.024
Sun Z, Li C, Wu D (2010) Removal of methylene blue from aqueous solution by adsorption onto zeolite synthesized from coal fly ash and its thermal regeneration. J Chem Technol Biotechnol 85:845–850. https://doi.org/10.1002/JCTB.2377
Sivalingam S, Kella T, Maharana M, Sen S (2019) Efficient sono-sorptive elimination of methylene blue by fly ash-derived nano-zeolite X: process optimization, isotherm and kinetic studies. J Clean Prod 208:1241–1254. https://doi.org/10.1016/J.JCLEPRO.2018.10.200
Inada M, Eguchi Y, Enomoto N, Hojo J (2005) Synthesis of zeolite from coal fly ashes with different silica–alumina composition. Fuel 84:299–304. https://doi.org/10.1016/J.FUEL.2004.08.012
Tabit K, Waqif M, Saâdi L (2019) Application of the Taguchi method to investigate the effects of experimental parameters in hydrothermal synthesis of Na-P1 zeolite from coal fly ash. Res Chem Intermed. https://doi.org/10.1007/s11164-019-03840-1
Sivalingam S, Sen S (2018) Optimization of synthesis parameters and characterization of coal fly ash derived microporous zeolite X. Appl Surf Sci 455:903–910. https://doi.org/10.1016/J.APSUSC.2018.05.222
Lu H, Zhang S, Li W et al (2017) Synthesis of graphene oxide-based sulfonated oligoanilines coatings for synergistically enhanced corrosion protection in 3.5% NaCl solution. ACS Appl Mater Interfaces 9:4034–4043. https://doi.org/10.1021/acsami.6b13722
Gooding OW (2004) Process optimization using combinatorial design principles: parallel synthesis and design of experiment methods. Curr Opin Chem Biol 8:297–304. https://doi.org/10.1016/J.CBPA.2004.04.009
Wu Q, Zhao G, Feng C et al (2011) Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples. J Chromatogr A 1218:7936–7942. https://doi.org/10.1016/J.CHROMA.2011.09.027
Al-Douri Y, Khan MM, Jennings JR (2023) Synthesis and optical properties of II–VI semiconductor quantum dots: a review. J Mater Sci 34:1–24. https://doi.org/10.1007/S10854-023-10435-5
Gimeno D (2011) Synthesis and characterization of Na-X, Na-A and Na-P zeolites and hydroxysodalite from metakaolinite. Clay Miner. https://doi.org/10.1180/claymin.2011.046.3.339
Carrado KA, Thiyagarajan P, Song K (1997) A study of organo-hectorite clay crystallization. Clay Miner 32:29–40. https://doi.org/10.1180/CLAYMIN.1997.032.1.05
Lev S, Zevin GK (1995) Quantitative X-ray diffractometry. Springer, Israel
Mintova S, Valtchev V (2002) Effect of the silica source on the formation of nanosized silicalite-1: an in situ dynamic light scattering study. Microporous Mesoporous Mater 55:171–179. https://doi.org/10.1016/S1387-1811(02)00401-8
Agudelo J, Hensen E, Giraldo S, Hoyos L (2015) Influence of steam-calcination and acid leaching treatment on the VGO hydrocracking performance of faujasite zeolite. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2015.01.011
Kazemian H, Naghdali Z, Ghaffari Kashani T, Farhadi F (2010) Conversion of high silicon fly ash to Na-P1 zeolite: alkaline fusion followed by hydrothermal crystallization. Adv Powder Technol 21:279–283. https://doi.org/10.1016/j.apt.2009.12.005
Li X, Han S, Xu J, Jiang N (2023) Green synthesis of nano-H-ZSM-5 zeolite single-crystal aggregates via an in situ reconstruction of the topology of natural clay. Microporous Mesoporous Mater 350:112441. https://doi.org/10.1016/J.MICROMESO.2023.112441
Alkan M, Hopa Ç, Yilmaz Z, Güler H (2005) The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater 86:176–184. https://doi.org/10.1016/J.MICROMESO.2005.07.008
Musyoka NM, Missengue R, Kusisakana M, Petrik LF (2014) Conversion of South African clays into high quality zeolites. Appl Clay Sci 97–98:182–186. https://doi.org/10.1016/J.CLAY.2014.05.026
Huang Y, Wang K, Dong D et al (2010) Synthesis of hierarchical porous zeolite NaY particles with controllable particle sizes. Microporous Mesoporous Mater 127:167–175. https://doi.org/10.1016/J.MICROMESO.2009.07.026
Chen D, Hu X, Shi L et al (2012) Synthesis and characterization of zeolite X from lithium slag. Appl Clay Sci 59–60:148–151. https://doi.org/10.1016/J.CLAY.2012.02.017
Dodin M, Paillaud J-L, Lorgouilloux Y et al (2010) A zeolitic material with a three-dimensional pore system formed by straight 12- and 10-ring channels synthesized with an imidazolium derivative as structure-directing agent. J Am Chem Soc 132:10221–10223. https://doi.org/10.1021/ja103648k
Nibou D, Mekatel H, Amokrane S et al (2010) Adsorption of Zn2+ ions onto NaA and NaX zeolites: kinetic, equilibrium and thermodynamic studies. J Hazard Mater 173:637–646. https://doi.org/10.1016/J.JHAZMAT.2009.08.132
Goswami M, Phukan P (2017) Enhanced adsorption of cationic dyes using sulfonic acid modified activated carbon. J Environ Chem Eng 5:3508–3517. https://doi.org/10.1016/j.jece.2017.07.016
Acknowledgements
The authors gratefully acknowledge the help provided by the Center of Analysis and Characterization (CAC) and Innovation Center (IC) at Caddy Ayyad University (Marrakech, Morocco).
Funding
The research, writing, and/or publishing of this paper were not supported financially by the authors in any way.
Author information
Authors and Affiliations
Contributions
AAB handled all the experiments and wrote the main manuscript text. KT and RI corrected the manuscript. With the material analysis, BD was a big assistance. The present effort was under the supervision of MEK and AA. The article has been considered by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors claim to be free of any financial or personal affiliations that may have seemed to have influenced the findings provided in this paper.
Ethical Approval
Not applicable (as the results of studies do not involve any human or animal).
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
baha, A.A., Tabit, K., Idouhli, R. et al. Effect of Liquor/(Si/Al) Ratio on Zeolite Synthesis from Fumed Silica and Coal Fly Ash Using the Taguchi Approach. Chemistry Africa 7, 1053–1062 (2024). https://doi.org/10.1007/s42250-023-00805-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00805-1