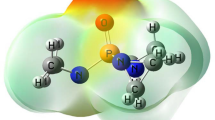
Abstract
The carbon nanotube’s (C56H16) stability and drug delivery capacity have already been investigated. The interaction of carbon nanotube C56H16 with superhalogen BF4 and superakali Na2F is studied using density functional theory (DFT), which resulted in the formation of the Na2F-CNT@BF4 endohedral complex. QTAIM analysis is used to calculate the nonbonding interactions (NICS) in Na2F-CNT@BF4 at the bond critical point (BCP). The charge transfer from BF4@CNT to superalkali entity Na2F has been observed, stabilizing Na2F-CNT@BF4. The calculated intensity as well as assignments of infrared spectra of CNT@BF4 and electronic transitions are compared with Na2F-CNT@BF4, which provides CNT’s polarization properties via interactions with Na2F and BF4. The charge transfer from CNT@BF4 to Na2F results in a large dipole moment (7.8969 D) of the complex, implying that its polarizability (588.4983 au) is comparable to that of CNT@BF4; however, the hyperpolarizability (4551.9858 au) of Na2F-CNT@BF4 is much higher than that of BF4@CNT. In this way, we hope that Na2F-CNT@BF4 will pique the interest of researchers interested in expanding the electro-optical applications of Na2F-CNT@BF4.





Similar content being viewed by others
Data availability
Data will be made available on reasonable request.
References
Tiwari SK, Kumar V, Huczko A, Oraon R, Adhikari AD, Nayak GC (2016) Crit Rev Sol State Mat Sci 41:257–317
Jabbar ML, Kadhim KJ (2021) Russ J Phys Chem B 15:46–52
Iijima S, Ichihashi T (1993) Nature 363(6430):603–605
Iijima S (1991) Nature 354(6348):56–58
Dresselhaus MS, Dresselhaus G, Avouris A, Rao AM (2000) Carbon Nanotubes. Springer, Berlin, pp 331–379
Mastronardo E, Bonaccorsi L, Kato Y, Piperopoulos E, Lanza M, Milone C (2016) Appl Energy 181:232–243
Yu MF, Files BS, Arepalli S, Ruoff RS (2000) Phys Rev Lett 84(24):5552–5555
**e Y, Wang T, Zhu B, Yan C, Zhang P, Wang X, Eres G (2018) Carbon 139:445–458
Yilmazoglu O, Yadav S, Cicek D, Schneider JJ (2016) Nanotechnology 27:365502 (1–10)
Kamegawa T (2009) Shokubai 51:368–372
**e S, Li W, Pan Z, Chang B, Lianfeng S (2000) J Phys Chem Sol 61:1153–1158
Fatemi SM, Foroutan M (2016) Int J Environ Sci Technol 13:457–470
Mashkoor F, Nasar A, Inamuddin A (2020) Environ Chem Lett 18:605–629
Ahmad J, Naeem S, Ahmad M, Usman ARA, Al-Wabel MIA (2019) J Environ Manag 246:214–228
Luo Y, Wang K, Li Q, Fan S, Wang J (2020) Small 16:1902719 (1–13)
Banerjee S, Hemraj-Benny T, Wong SS (2005) Adv Mater 17:17–29
Hemraj-Benny T, Banerjee S, Wong SS (2004) Chem Mater 16(10):1855–1863
Bomboi F, Bonincontro A, La Mesa C, Tardani F (2011) J Colloid Int Sci 355(2):342–347
Luong JHT, Hrapovic S, Wang D, Bensebaa F, Simard B (2004) Electroanalysis 16(1–2):132–139
Rybolt T, Jordan H (2021) Appl Nano Mater 2:128–147
Chiou K, Huang J (2020) Matter 3:302–319
Kosar N, Wajid S, Ayub K, Mahmood T (2023) Optik 276:170660
Asif M, Sajid H, Ayub K, Gilani MA, Mahmood T (2022) Polyhedron 215:115695
Yaqoob J, Mahmood T, Ayub K, Tabassum S, Khan AF, Perveen S, Yang J, Gilani MA (2022) Euro Phys J Plus 137:233
Gul S, Bhatti IA, Ayub K, Shawky AM, Iqbal MAJ (2022) Alkali metal (Na and Li) doped C18 nanocluster with boosted electronic and nonlinear optical properties. Optik 271:170185
Gutsev GL, Bartlett RJ, Boldyrev AI, Simons J (1997) J Chem Phys 107:3867–3875
Gutsev GL, Boldyrev AI (1982) Chem Phys Lett 92:262–266
Rehm E, Boldyrev AI, Schleyer PVR (1992) Inorg Chem 31:4834–4842
Srivastava AK, Srivastava H, Tiwari A, Misra N (2022) Chem Phys Lett 790:139352
Yang H, Li Y, Wu D, Li ZR (2012) Int J Quant Chem 112:770–778
Li Y, Wu D, Li ZR (2008) Inorg Chem 47:9773–9778
Srivastava AK, Kumar A, Misra N (2017) Chem Phys Lett 682:20–25
Wang FF, Li ZR, Wu D, Sun XY, Chen W, Li Y, Sun CC (2006) ChemPhysChem 7:1136–1141
Aoyagi S, Nishibori E, Sawa H et al (2010) Nat Chem 2:678–683
Okada H, Komuro T, Sakai T et al (2012) RSC Adv 2:10624–10631
Wang S-J, Li Y, Wang Y-F, Wu D, Li Z-R (2013) Phys Chem Chem Phys 15:12903–12910
Srivastava AK, Kumar A, Misra N (2016) Physica E Low Dimens Syst Nanostruct 84:524–529
Pandey AK, Singh V, Dwivedi A (2021) NANO 16(09):2150106
Pandey AK, Dwivedi A, Mishra AK, Tiwari SN, Vuai SAH, Singh V (2023) J Ind Chem Soc 100:100851
Srivastava AK, Misra N (2016) Mol Sim 42(12):981–985
Frisch MJ, Trucks GW, Schlegel HB et al (2010) Gaussian 09, Revision C02. Gaussian Inc., Wallingford
Becke AD (1988) Phys Rev A 38:3098–3100 (Lee C, Yang W, Parr RG (1988) Phys. Rev. B 37:785-789)
Sikorska C, Puzyn T (2015) Nanotechnology 26:455702 (1–10)
Srivastava AK, Pandey SK, Misra N (2016) Chem Phy Lett 655–656:71–75
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Frisch A, Nelson AB, Holder AJ (2000) GAUSSVIEW Inc., Pittsburgh
Keith TA, Gristmill TK (2012) AIMAll (Version 12.09.23), Overland Park KS, USA
Padrón JA, Carasco R, Pellón RF (2002) J Pharm Pharm Sci 5:258–266
Verma RP, Hansch C (2005) Bioorg Med Chem 13:2355–2372
Verma RP, Kurup A, Hansch C (2005) Bioorg Med Chem 13:237–255
Vuks MF (1966) Opt Spectrosc 20:361–368
Kleinmann DA (1962) Phys Rev 126:1977–1979
Tavakol H, Haghshenas H (2021) Quantum Rep 3:366–375
Srivastava AK, Misra N (2015) Polyhedron 102:711–714
Srivastava AK, Kumar A, Misra N (2017) J Fluor Chem 197:59–62
Bader RFW (1990) Atoms in Molecules: A Quantum Theory, 2nd edn. Oxford, New York
Bader RFW (1991) Chem Rev 91:893–928
Koch U, Popelier P (1995) J Phys Chem A 99:9747–9754
Cremer D, Kraka E (1984) Croat Chem Acta 57:1259–1281
Bader RFW, Essen H (1984) J Chem Phys 80:1943–1960
Espinosa E, Monils E, Lecomte C (1998) Chem Phys Lett 285:170–173
Pearson RG (1985) J Am Chem Soc 107:6801–6806
Khan MZH, Alrawashdeh AI, Aljohani S, Zhao Y, Lagowski JB (2017) Phys Chem Chem Phys 41:1–12
Omidvar A, Anafcheh M, Hadipour NL (2013) Scientia Iranica Trans F Nanotechnol 20:1014–1017
Koopmans TA (1933) Physica 91:104–113
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Parr RG, Szentpaly LV, Liu SJ (1999) Am Chem Soc 121:1922–1924
Padmanabhan J, Parthasarathi R, Subramaniaan V, Chattaraj PK (2007) J Phys Chem A 111007:1358–1361
Antoine R, Dugourd P, Rayane D, Benichou E, Broyer M, Chandezon F, Guet C (1999) J Chem Phys 110:9771–9772
Champagne B, Perpete EA, Jacquemin D, van Gisbergen SJA, Baerends EJ, Soubra-Ghaoui C, Robins KA, Kirtman B (2000) J Phys Chem A 104:4755–4763
Peach MJG, Helgaker T, Salek P, Keal TW, Lutnaes OB, Tozer DJ, Handy NC (2006) Phys Chem Chem Phys 8:558–562
Cai Z-L, Crossley MJ, Reimers JR, Kobayashi R, Amos RD (2006) J Phys Chem B 110:15624–15632
Zhang CR, Chen HS, Wang GH (2004) Structure and properties of semiconductor Microclusters GanPn(n = 1–4): a first principle study. Chem Res Chin Univ 20(5):640–643
Colthup NB, Daly LH, Wiberley SE (1990) Introduction to Infrared and Raman Spectroscopy, 3rd edn. Academic Press, Boston
Kosar N, Ayub K, Mahmood T (2021) J Mol Graph Mod 102:107794 (1–12)
Khan P, Mahmood P, Ayub K, Sobia T, Gilani MA (2021) Opt Laser Technol 142:107231 (1–10)
Mahmood A, Abdullah MI (2015) Khan SU-D. Spectrochem Acta Part A Mol Biomol Spectrosc 139:425–430
Kanis DR, Ratner MA, Marks TJ (1994) Chem Rev 94:195–242
Acknowledgements
V.S. is grateful and acknowledges the computer resources, technical expertise, and assistance provided by the Center for High-Performance Computing (MATS1467) Cape Town, South Africa.
Funding
Author Anoop Kumar Pandey is grateful and thanks to the Uttar Pradesh government (India) [No:46/2021/603/sattar-4-2021-4(56)/2020] for providing him with financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all the authors. The first draft of the manuscript was written by VS, AD and AKP, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, V., Dwivedi, A., Mishra, A.K. et al. Variation in Structural Chemical Reactivity and Nonlinear Optical (NLO) Properties of C56H16 Nanotube After Endohedral Do** of Superalkali (Na2F) and Superhalogen (BF4): A DFT Study. Chemistry Africa 7, 315–327 (2024). https://doi.org/10.1007/s42250-023-00739-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00739-8