Abstract
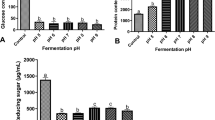
The food value of cassava is limited because of the presence of cyanogenic glucosides (linamarin and lotaustralin), which when hydrolyzed by the endogenous enzyme linamarase, produce acetone cyanohydrin and hydrogen cyanide. This research is aimed to evaluate how inoculum size affects the solid-state fermentation of cassava by the fungus R. Oligosporus. The enzymatic activities and biochemical parameters were measured using spectrophotometer and the colorimetric approach. The supernatant of the R. Oligosporus was used as the crude extract or sample for the various assays that includes test for total amylase activity, glucose and total soluble proteins. The results for Amylase activity show that after 72 h, amylase activity increased significantly (p < 0.05) in the unfermented control (0%) at 20% inoculum size. Meanwhile, at different inoculum sizes, there was also a rise in amylase activity. Furthermore, the glucose analysis revealed that after 72 h of fermentation, the glucose concentration, which was low in the control, had considerably increased (P < 0.05). In addition, an increase in soluble proteins was also observed at all inoculum size fermented and both the unfermented control. The findings of the current research showed that solid state fermentation of cassava with the fungus R. Oligosporus increased nutritional value (proteins and glucose) and nutrient bioavailability at all inoculum sizes studied.



Similar content being viewed by others
Change history
16 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s42250-023-00752-x
References
Anikwe MAN, Ikenganyia EE (2018) Ecophysiology and production principles of cassava (Manihot species) in Southeastern Nigeria. Cassava, InTech. https://doi.org/10.5772/intechopen.70828
Imakumbili MLE, Semu E, Semoka JMR, Abass A, Mkamilo G (2019) Farmers’ perceptions on the causes of cassava root bitterness: a case of konzo-affected Mtwara region, Tanzania. PLoS One 14:e0215527. https://doi.org/10.1371/journal.pone.0215527
Uritu CM, Mihai CT, Stanciu G-D, Dodi G, Alexa-Stratulat T, Luca A, Leon-Constantin M-M, Stefanescu R, Bild V, Melnic S, Tamba BI (2018) Medicinal plants of the family Lamiaceae in pain therapy: a review. Manag Pain Res. https://doi.org/10.1155/2018/7801543
Edo GI, Onoharigho FO, Akpoghelie PO, Emakpor OL, Ozgor E, Akhayere E (2022) Physicochemical, phytochemical, antioxidant, and inhibition properties of key enzymes linked to raw and regular honey. Chem Africa. https://doi.org/10.1007/s42250-022-00401-9
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25:361–366. https://doi.org/10.1016/j.sjbs.2017.02.004
Lizardi-Jiménez MA, Hernández-Martínez R (2017) Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. https://doi.org/10.1007/s13205-017-0692-y
Freitas A, Escaramboni B, Carvalho A, Lima V, Oliva-Neto P (2014) Production and application of amylases of Rhizopus oryzae and Rhizopus microsporus var. oligosporus from industrial waste in acquisition of glucose. Chem Pap. https://doi.org/10.2478/s11696-013-0466-x
Edo GI (2022) Antibacterial, phytochemical and GC-MS analysis of thevetia peruviana extracts: an approach in drug formulation. Nat Resour Hum Heal. https://doi.org/10.53365/nrfhh/146543
Nyirenda KK (2021) Toxicity potential of cyanogenic glycosides in edible plants. Toxicol IntechOpen Med. https://doi.org/10.5772/intechopen.91408
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG (2017) Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol. https://doi.org/10.3389/fmicb.2016.02170
Zidenga T, Siritunga D, Sayre RT (2017) Cyanogen metabolism in cassava roots: impact on protein synthesis and root development. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00220
Samtiya M, Aluko RE, Dhewa T (2020) Plant food anti-nutritional factors and their reduction strategies: an overview, food prod. Process Nutr 2:6. https://doi.org/10.1186/s43014-020-0020-5
Dereje B, Girma A, Mamo D, Chalchisa T (2020) Functional properties of sweet potato flour and its role in product development: a review. Int J Food Prop 23:1639–1662. https://doi.org/10.1080/10942912.2020.1818776
Cooke RD, Maduagwu EN (2007) The effects of simple processing on the cyanide content of cassava chips. Int J Food Sci Technol 13:299–306. https://doi.org/10.1111/j.1365-2621.1978.tb00807.x
Njankouo Ndam Y, Mounjouenpou P, Kansci G, Kenfack MJ, Fotso Meguia MP, Natacha Ngono Eyenga NS, Mikhaïl Akhobakoh M, Nyegue A (2019) Influence of cultivars and processing methods on the cyanide contents of cassava (Manihot esculenta Crantz) and its traditional food products. Sci Afr 5:e00119. https://doi.org/10.1016/j.sciaf.2019.e00119
Lee GY, Li W, Chirwa UM, Shi J (2020) Effect of substrate characteristics on the growth and sporulation of two biocontrol microorganisms during solid state cultivation. Fermentation 6:69. https://doi.org/10.3390/fermentation6030069
Jain A, Jain R, Jain S (2020) Determination of salivary amylase activity. Springer, Berlin, pp 223–225. https://doi.org/10.1007/978-1-4939-9861-6_52
Rhee MK, Ho Y-L, Raghavan S, Vassy JL, Cho K, Gagnon D, Staimez LR, Ford CN, Wilson PWF, Phillips LS (2019) Random plasma glucose predicts the diagnosis of diabetes. PLoS One 14:e0219964. https://doi.org/10.1371/journal.pone.0219964
Kusumiyati Y, Hadiwijaya IE, Putri S, Mubarok JS, Hamdani (2020) Rapid and non-destructive prediction of total soluble solids of guava fruits at various storage periods using handheld near-infrared instrument, IOP Conf. Ser Earth Environ Sci 458:012022. https://doi.org/10.1088/1755-1315/458/1/012022
Raul D, Biswas T, Mukhopadhyay S, Kumar Das S, Gupta S (2014) Production and partial purification of alpha amylase from Bacillus subtilis (MTCC 121) using solid state fermentation. Biochem Res Int 2014:1–5. https://doi.org/10.1155/2014/568141
Adebo OA, Gabriela Medina-Meza I (2020) Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: a mini review. Molecules 25:927. https://doi.org/10.3390/molecules25040927
Onyibe PN, Edo GI, Nwosu LC, Ozgor E (2021) Effects of vernonia amygdalina fractionate on glutathione reductase and glutathione-S-transferase on alloxan induced diabetes wistar rat. Biocatal Agric Biotechnol 36:102118. https://doi.org/10.1016/j.bcab.2021.102118
Nwosu LC, Edo GI, Ozgor E (2022) The phytochemical, proximate, pharmacological, GC-MS analysis of Cyperus esculentus (Tiger nut): a fully validated approach in health, food and nutrition. Food Biosci. https://doi.org/10.1016/j.fbio.2022.101551
Maicas S (2020) The role of yeasts in fermentation processes. Microorganisms 8:1142. https://doi.org/10.3390/microorganisms8081142
Swain MR, Anandharaj M, Ray RC, Parveen Rani R (2014) Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol Res Int 2014:1–19. https://doi.org/10.1155/2014/250424
Hawashi M, Widjaja T, Gunawan S (2020) Solid-State fermentation of cassava products for degradation of anti-nutritional value and enrichment of nutritional value. Ferment Process IntechOpen New Adv. https://doi.org/10.5772/intechopen.87160
Hassan F, Edo GI, Nwosu LC, Jalloh AA, Onyibe PN, Itoje-akpokiniovo LO, Irogbo PU (2021) An inventory of medicinal plants used as sedative, analgesic and blood tonic in Abeokuta, Ogun State, Nigeria. Acta Ecol Sin. https://doi.org/10.1016/j.chnaes.2021.11.003
Edo GI (2022) Effects of paraquat dichloride on adult male wistar rat. an approach in the toxicity of body weights and hematological tissues. J Anal Pharm Res 11:1–7. https://doi.org/10.15406/japlr.2022.11.00394
Tonukari NJ, Tonukari NJ, Ezedom T, Enuma CC, Sakpa SO, Avwioroko OJ, Eraga L, Odiyoma E (2015) White gold: cassava as an industrial base. Am J Plant Sci 06:972–979. https://doi.org/10.4236/ajps.2015.67103
Gopinath SCB, Anbu P, Arshad MKM, Lakshmipriya T, Voon CH, Hashim U, Chinni SV (2017) Biotechnological processes in microbial amylase production. Biomed Res Int 2017:1–9. https://doi.org/10.1155/2017/1272193
Nkhata SG, Ayua E, Kamau EH, Shingiro J-B (2018) Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci Nutr 6:2446–2458. https://doi.org/10.1002/fsn3.846
do Nascimento FV, Lemes AC, de Castro AM, Secchi AR, Zarur Coelho MA, (2022) A temporal evolution perspective of lipase production by Yarrowia lipolytica in solid-state fermentation. Processes. 10:381. https://doi.org/10.3390/pr10020381
Manan MA, Webb C (2020) Newly designed multi-stacked circular tray solid-state bioreactor: analysis of a distributed parameter gas balance during solid-state fermentation with influence of variable initial moisture content arrangements. Bioresour Bioprocess 7:16. https://doi.org/10.1186/s40643-020-00307-9
Poggio D, Walker M, Nimmo W, Ma L, Pourkashanian M (2016) Modelling the anaerobic digestion of solid organic waste – substrate characterisation method for ADM1 using a combined biochemical and kinetic parameter estimation approach. Waste Manag 53:40–54. https://doi.org/10.1016/j.wasman.2016.04.024
Gougoulias C, Clark JM, Shaw LJ (2014) The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J Sci Food Agric 94:2362–2371. https://doi.org/10.1002/jsfa.6577
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01617
Soccol CR, da Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, de Vandenberghe LP (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1:52–71. https://doi.org/10.1016/j.biori.2017.01.002
Acknowledgements
The authors wish to thank Mr Joseph Sani Sahjury (department of Information Technology) for SPSS training during this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EOE, GIE, AAA, GE, NJT: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—Original Draft, Visualization, Supervision, Project administration. FOO: Conceptualization, Formal analysis, Investigation, Writing—Original Draft, Visualization. OLE: Conceptualization, Formal analysis, Investigation, Writing—Original Draft, Visualization. EO: Conceptualization, Formal analysis, Investigation, Writing—Original Draft, Visualization. EA: Conceptualization, Formal analysis, Investigation, Writing—Original Draft, Visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Not all authors were listed in the article.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Egbune, E.O., Anigboro, A.A., Edeche, G. et al. Effect of Inoculum Size on Solid State Fermentation of Cassava (Manito esculenta Crantz). Chemistry Africa 6, 2911–2917 (2023). https://doi.org/10.1007/s42250-022-00434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00434-0