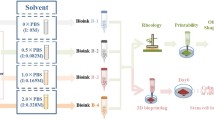
Abstract
Bioprinting has been widely investigated for tissue engineering and regenerative medicine applications. However, it is still difficult to reconstruct the complex native cell arrangement due to the limited printing resolution of conventional bioprinting techniques such as extrusion- and inkjet-based printing. Recently, an electrohydrodynamic (EHD) bioprinting strategy was reported for the precise deposition of well-organized cell-laden constructs with microscale filament size, whereas few studies have been devoted to develo** bioinks that can be applied for EHD bioprinting and simultaneously support cell spreading. This study describes functionalized alginate-based bioinks for microscale EHD bioprinting using peptide grafting and fibrin incorporation, which leads to high cell viability (>90%) and cell spreading. The printed filaments can be further refined to as small as 30 μm by incorporating polyoxyethylene and remained stable over one week when exposed to an aqueous environment. By utilizing the presented alginate-based bioinks, layer-specific cell alignment along the printing struts could be observed inside the EHD-printed microscale filaments, which allows fabricating living constructs with cell-scale filament resolution for guided cellular orientation.
Graphic abstract







Similar content being viewed by others
References
Vijayavenkataraman S, Yan WC, Lu WF et al (2018) 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev 132:296–332. https://doi.org/10.1016/j.addr.2018.07.004
Aljohani W, Ullah MW, Zhang X et al (2017) Bioprinting and its applications in tissue engineering and regenerative medicine. Int J Biol Macromol 107(Part A):261–275. https://doi.org/10.1016/j.ijbiomac.2017.08.171
Mota C, Camarero-Espinosa S, Baker MB et al (2020) Bioprinting: from tissue and organ development to in vitro models. Chem Rev 120(19):10547–10607. https://doi.org/10.1021/acs.chemrev.9b00789
Zhu H, Monavari M, Zheng K et al (2022) 3D bioprinting of multifunctional dynamic nanocomposite bioinks incorporating Cu-doped mesoporous bioactive glass nanoparticles for bone tissue engineering. Small 18(12):e2104996. https://doi.org/10.1002/smll.202104996
Li X, Liu B, Pei B et al (2020) Inkjet bioprinting of biomaterials. Chem Rev 120(19):10793–10833. https://doi.org/10.1021/acs.chemrev.0c00008
Dou C, Perez V, Qu J et al (2021) A state-of-the-art review of laser-assisted bioprinting and its future research trends. ChemBioEng Rev 8(5):517–534. https://doi.org/10.1002/cben.202000037
Heid S, Becker K, Byun J et al (2022) Bioprinting with bioactive alginate dialdehyde-gelatin (ADA-GEL) composite bioinks: time-dependent in-situ crosslinking via addition of calcium-silicate particles tunes in vitro stability of 3D bioprinted constructs. Bioprinting 26:e00200. https://doi.org/10.1016/j.bprint.2022.e00200
Moroni L, Burdick JA, Highley C et al (2018) Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater 3(5):21–37. https://doi.org/10.1038/s41578-018-0006-y
Brassard JA, Lutolf MP (2019) Engineering stem cell self-organization to build better organoids. Cell Stem Cell 24(6):860–876. https://doi.org/10.1016/j.stem.2019.05.005
**ng J, Liu N, Xu N et al (2021) Engineering complex anisotropic scaffolds beyond simply uniaxial alignment for tissue engineering. Adv Funct Mater 32(15):2110676. https://doi.org/10.1002/adfm.202110676
Cui C, Yang C, Eidson N et al (2020) A highly reversible, dendrite-free lithium metal anode enabled by a lithium-fluoride-enriched interphase. Adv Mater 32(12):e1906427. https://doi.org/10.1002/adma.201906427
Choi SW, Choi Y, Kim J (2019) In situ magnetic alignment and cross-linking of injectable microparticles into centimeter-scale fibers for efficient myoblast alignment and in vivo fiber formation. Chem Mater 31(14):5181–5189. https://doi.org/10.1021/acs.chemmater.9b01276
Jana S, Levengood SK, Zhang M (2016) Anisotropic materials for skeletal-muscle-tissue engineering. Adv Mater 28(48):10588–10612. https://doi.org/10.1002/adma.201600240
Zhuang P, An J, Chua CK et al (2020) Bioprinting of 3D in vitro skeletal muscle models: a review. Mater Des 193:108794. https://doi.org/10.1016/j.matdes.2020.108794
Zhang Y, Zhang Z, Wang Y (2020) 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for skeletal muscle tissue engineering. Mater Sci Eng C Mater Biol Appl 116:111070. https://doi.org/10.1016/j.msec.2020.111070
Charest JL, García AJ, King WP (2007) Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials 28(13):2202–2210. https://doi.org/10.1016/j.biomaterials.2007.01.020
Mao M, He J, Li Z et al (2020) Multi-directional cellular alignment in 3D guided by electrohydrodynamically-printed microlattices. Acta Biomater 101:141–151. https://doi.org/10.1016/j.actbio.2019.10.028
**g L, Sun J, Liu H et al (2021) Using plant proteins to develop composite scaffolds for cell culture applications. Int J Bioprint 7(1):298. https://doi.org/10.18063/ijb.v7i1.298
Zhang B, He J, Li X et al (2016) Micro/nanoscale electrohydrodynamic printing: from 2D to 3D. Nanoscale 8(34):15376–15388. https://doi.org/10.1039/c6nr04106j
Ye W, **e C, Liu Y et al (2021) 3D printed high-resolution scaffold with hydrogel microfibers for providing excellent biocompatibility. J Biomater Appl 35(6):633–642. https://doi.org/10.1177/0885328220962606
Gao Q, **e C, Wang P et al (2020) 3D printed multi-scale scaffolds with ultrafine fibers for providing excellent biocompatibility. Mater Sci Eng C Mater Biol Appl 107:110269. https://doi.org/10.1016/j.msec.2019.110269
Wang C, Xu Y, **a J et al (2021) Multi-scale hierarchical scaffolds with aligned micro-fibers for promoting cell alignment. Biomed Mater 16(4):045047. https://doi.org/10.1088/1748-605X/ac0a90
Hu S, Meng Z, Zhou J et al (2022) Enhanced attachment and collagen type I deposition of MC3T3-E1 cells via electrohydrodynamic printed sub-microscale fibrous architectures. Int J Bioprint 8(2):514. https://doi.org/10.18063/ijb.v8i2.514
He J, Hao G, Meng Z et al (2021) Expanding melt-based electrohydrodynamic printing of highly-ordered microfibrous architectures to cm-height via in situ charge neutralization. Adv Mater Technol 7(7):2101197. https://doi.org/10.1002/admt.202101197
He J, Zhao X, Chang J et al (2017) Microscale electro-hydrodynamic cell printing with high viability. Small 13(47):1702626. https://doi.org/10.1002/smll.201702626
Sampson SL, Saraiva L, Gustafsson K (2014) Cell electrospinning: an in vitro and in vivo study. Small 10(1):78–82. https://doi.org/10.1002/smll.201300804
Yeo M, Kim G (2020) Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater 107:102–114. https://doi.org/10.1016/j.actbio.2020.02.042
Yeo M, Ha J, Lee H et al (2016) Fabrication of hASCs-laden structures using extrusion-based cell printing supplemented with an electric field. Acta Biomater 38:33–43. https://doi.org/10.1016/j.actbio.2016.04.017
Yeo M, Kim GH (2018) Anisotropically aligned cell-laden nanofibrous bundle fabricated via cell electrospinning to regenerate skeletal muscle tissue. Small 14(48):e1803491. https://doi.org/10.1002/smll.201803491
Castilho M, Levato R, Bernal PN et al (2021) Hydrogel-based bioinks for cell electrowriting of well-organized living structures with micrometer-scale resolution. Biomacromol 22(2):855–866. https://doi.org/10.1021/acs.biomac.0c01577
Galliger Z, Vogt CD, Panoskaltsis-Mortari A (2019) 3D bioprinting for lungs and hollow organs. Transl Res 211:19–34. https://doi.org/10.1016/j.trsl.2019.05.001
Ballester-Beltrán J, Lebourg M, Rico P et al (2012) Dorsal and ventral stimuli in cell-material interactions: effect on cell morphology. Biointerphases 7:39. https://doi.org/10.1007/s13758-012-0039-5
Bauer A, Gu L, Kwee B et al (2017) Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater 62:82–90. https://doi.org/10.1016/j.actbio.2017.08.041
Vion AC, Perovic T, Petit C et al (2021) Endothelial cell orientation and polarity are controlled by shear stress and VEGF through distinct signaling pathways. Front Physiol 11:623769. https://doi.org/10.3389/FPHYS.2020.623769
Finch DA, Ralph B, Gilding K (1997) Determination of the cation content of alginate thin films by FTi.r. spectroscopy. Polymer 38(1):43–51. https://doi.org/10.1016/S0032-3861(96)00458-2
Tenchurin TK, Pavlovsky MM, Shepelev AD et al (2019) Modification of non-woven materials based on sodium alginate for tissue-engineering. J Phys Conf Ser 1347:012072. https://doi.org/10.1088/1742-6596/1347/1/012072
Sun J, Wei D, Yang K et al (2017) The development of cell-initiated degradable hydrogel based on methacrylated alginate applicable to multiple microfabrication technologies. J Mater Chem B 5(40):8060–8069. https://doi.org/10.1039/c7tb01458a
Du J, Zhou C, **a Q et al (2022) The effect of fibrin on rheological behavior, gelling properties and microstructure of myofibrillar proteins. LWT 153:112457. https://doi.org/10.1016/j.lwt.2021.112457
Vicar T, Balvan J, Jaros J (2019) Cell segmentation methods for label-free contrast microscopy: review and comprehensive comparison. BMC Bioinform 20:360. https://doi.org/10.1186/s12859-019-2880-8
Indana D, Agarwal P, Bhutani N et al (2021) Viscoelasticity and adhesion signaling in biomaterials control human pluripotent stem cell morphogenesis in 3D culture. Adv Mater 33(43):e2101966. https://doi.org/10.1002/adma.202101966
Bidarra SJ, Barrias CC, Granja PL (2014) Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater 10(4):1646–1662. https://doi.org/10.1016/j.actbio.2013.12.006
Tiwari SK, Venkatraman SS (2012) Importance of viscosity parameters in electrospinning: of monolithic and core–shell fibers. Mater Sci Eng C 32(5):1037–1042. https://doi.org/10.1016/j.msec.2012.02.019
Kim MW, Cao BH (1993) Additional reduction of surface tension of aqueous polyethylene oxide (PEO) solution at high polymer concentration. EPL 24(3):229–234. https://doi.org/10.1209/0295-5075/24/3/012
Liashenko I, Rosell-Llompart J, Cabot A (2020) Ultrafast 3D printing with submicrometer features using electrostatic jet deflection. Nat Commun 11(1):753. https://doi.org/10.1038/s41467-020-14557-w
Zhao X, He J, Xu F et al (2016) Electrohydrodynamic printing: a potential tool for high-resolution hydrogel/cell patterning. Virtual Phys Prototyp 11(1):57–63. https://doi.org/10.1080/17452759.2016.1139378
Lee JM, Yeong WY (2020) Engineering macroscale cell alignment through coordinated toolpath design using support-assisted 3D bioprinting. J R Soc Interface 17(168):20200294. https://doi.org/10.1098/rsif.2020.0294
Schwab A, Levato R, D’Este M (2020) Printability and shape fidelity of bioinks in 3D bioprinting. Chem Rev 120(19):11028–11055. https://doi.org/10.1021/acs.chemrev.0c00084
Aubin H, Nichol JW, Hutson CB et al (2010) Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 31(27):6941–6951. https://doi.org/10.1016/j.biomaterials.2010.05.056
Yang GH, Kim W, Kim J et al (2021) A skeleton muscle model using GelMA-based cell-aligned bioink processed with an electric-field assisted 3D/4D bioprinting. Theranostics 11(1):48–63. https://doi.org/10.7150/THNO.50794
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2018YFA0703003), the National Natural Science Foundation of China (No. 52125501), the Key Research Project of Shaanxi Province (Nos. 2021LLRH-08, 2020GXLH-Y-021, and 2021GXLH-Z-028), the Youth Innovation Team of Shaanxi Universities and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
ZNQ, YTW and AK were involved in the methodology and investigation; ZNQ contributed to the formal analysis; HZ assisted in writing—original draft; all authors participated in writing—review and editing; DCL and JKH contributed to the funding acquisition and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, Z., Zhu, H., Wang, Y. et al. Functionalized alginate-based bioinks for microscale electrohydrodynamic bioprinting of living tissue constructs with improved cellular spreading and alignment. Bio-des. Manuf. 6, 136–149 (2023). https://doi.org/10.1007/s42242-022-00225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-022-00225-z