Abstract
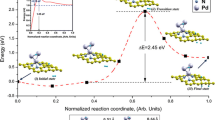
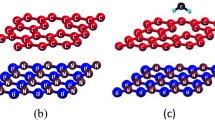
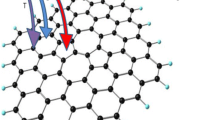
Adsorption and desorption of hydrogen on/from single-vacancy and double-vacancy graphenes were studied by means of first-principles calculations. The structure and stability of continuous hydrogenation in single vacancy were investigated. Several new stable structures were found, along with their corresponding energy barriers. In double-vacancy graphene, the preferred sites of H atoms were identified, and H2 molecule desorption and adsorption of from/on were calculated from the energy barriers. This work provides a systematic and comprehensive understanding of hydrogen behavior on defected graphene.






Similar content being viewed by others
References
I. Staffell, The energy and fuel data sheet. 2011. http://tinyurl.com/energydata
P.D. Jongh, M. Allendorf, J.J. Vajo et al., Nanoconfined light metal hydrides for reversible hydrogen storage. MRS Bull. 38, 488–494 (2013). https://doi.org/10.1557/mrs.2013.108
L. Pickering, J. Li, D. Reed et al., Ti–V–Mn based metal hydrides for hydrogen storage. J. Alloys Compd. 580, 233–237 (2013). https://doi.org/10.1016/j.jallcom.2013.03.208
A.M. Jorge, E. Prokofiev, G.L. de Lima et al., An investigation of hydrogen storage in a magnesium-based alloy processed by equal-channel angular pressing. Int. J. Hydrogen Energy 38, 8306–8312 (2013). https://doi.org/10.1016/j.ijhydene.2013.03.158
D.L. Chao, C.L. Zhong, Z.W. Ma et al., Improvement in high-temperature performance of Co-free high-Fe AB 5-type hydrogen storage alloys. Int. J. Hydrogen Energy 37, 12375–12383 (2012). https://doi.org/10.1016/j.ijhydene.2012.05.147
C.N. Peng, The effects of hydrogen on the helium behavior in palladium. Nucl. Sci. Tech. 27, 106 (2016). https://doi.org/10.1007/s41365-016-0115-5
W.G. Liu, Y. Qian, D.X. Zhang et al., Theoretical study of the interaction between hydrogen and 4d alloying atom in nickel. Nucl. Sci. Tech. 28, 82 (2017). https://doi.org/10.1007/s41365-017-0235-6
P. Reunchan, S.H. Jhi, Metal-dispersed porous graphene for hydrogen storage. Appl. Phys. Lett. 98, 93–103 (2011). https://doi.org/10.1063/1.3560468
Y. **a, Z. Yang, Y. Zhu, Porous carbon-based materials for hydrogen storage: advancement and challenges. J. Mater. Chem. A 1, 9365–9381 (2013). https://doi.org/10.1039/c3ta10583k
X.K. Chen, J. Liu, Z.H. Peng et al., A wave-dominated heat transport mechanism for negative differential thermal resistance in graphene/hexagonal boron nitride heterostructures. Appl. Phys. Lett. 110, 091907 (2017). https://doi.org/10.1063/1.4977776
X.K. Chen, Z.X. **e, W.X. Zhou et al., Thermal rectification and negative differential thermal resistance behaviors in graphene/hexagonal boron nitride heterojunction. Carbon 100, 492–500 (2016). https://doi.org/10.1016/j.carbon.2016.01.045
M. Pumera, Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 4, 668–674 (2011). https://doi.org/10.1039/c0ee00295j
S. Patchkovskii, J.S. Tse, S.N. Yurchenko et al., Graphene nanostructures as tunable storage media for molecular hydrogen. Natl. Acad. Sci. USA 102, 10439–10444 (2005). https://doi.org/10.1073/pnas.0501030102
D.W. Boukhvalov, M.I. Katsnelson, A.I. Lichtenstein, Hydrogen on graphene: electronic structure, total energy, structural distortions and magnetism from first-principles calculations. Phys. Rev. B 77, 035427 (2008). https://doi.org/10.1103/PhysRevB.77.035427
D.C. Elias, R.P. Nair, T.M. Mohiuddin et al., Control of grphene’s properties by reversible hydrogenation: evidence for graphane. Science 323, 610–613 (2009). https://doi.org/10.1126/science.1167130
R. Balog, B.J. Jorgensen, L. Nilsson et al., Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat. Mat. 9, 315–319 (2010). https://doi.org/10.1038/NMAT2710
B.S. Pujari, S. Gusarov, M. Brett et al., Single-side-hydrogenated graphene: density functional theory predictions. Phys. Rev. B 84, 041402 (2011). https://doi.org/10.1103/PhysRevB.84.041402
S.S. Han, H. Jung, D.H. Jung et al., Stability of hydrogenation states of graphene and conditions for hydrogen spillover. Phys. Rev. B 85, 155408 (2012). https://doi.org/10.1103/PhysRevB.85.155408
W. Zhou, J. Zhou, J. Shen et al., First-principles study of high-capacity hydrogen storage on graphene with Li atoms. J. Phys. Chem. Solids 73, 245–251 (2012). https://doi.org/10.1016/j.jpcs.2011.10.035
V. Tozzini, V. Pellegrini, Prospects for hydrogen storage in graphene. Phys. Chem. Chem. Phys. 15, 80–89 (2013). https://doi.org/10.1039/c2cp42538f
S. Gadipelli, Z. Guo, Graphene-based materials: synthesis and gas sorption, storage and separation. Prog. Mater Sci. 69, 1–60 (2015). https://doi.org/10.1016/j.pmatsci.2014.10.004
H. Hu, J. **n, H. Hu et al., Metal-free graphene-based catalyste-insight into the catalytic activity: a short review. Appl. Catal. A. Gen. 492, 1–9 (2015). https://doi.org/10.1016/j.apcata.2014.11.041
M.L. Guillou, N. Toulhoat, Y. Pipon et al., Deuterium migration in nuclear graphite: consequences for the behavior of tritium in CO2-cooled reactors and for the decontamination of irradiated graphite waste. J. Nucl. Mater. 461, 72–77 (2015). https://doi.org/10.1016/j.jnucmat.2015.03.005
T.N. Hoai, K.H. Lam, N.T. Thanh et al., Migration and desorption of hydrogen atom and molecule on/from graphene. Carbon 121, 248–256 (2017). https://doi.org/10.1016/j.carbon.2017.05.069
Y. Miura, H. Kasai, W. Dino et al., First principles studies for the dissociative adsorption of H2 on graphene. J. Appl. Phys. 933, 395–400 (2003). https://doi.org/10.1063/1.1555701
Z. Ao, S. Li, in Graphene Simulation, ed. by J.R. Gong (InTech, Rijeka, 2011), p. 53
M. Terrones, A.R. Botello-Mendez, J. Campos-Delgado et al., Graphene and graphite nanoribbons: morphology, properties, synthesis, defects and applications. Nano Today 5, 351–372 (2010). https://doi.org/10.1016/j.nantod.2010.06.010
K.S. Novoselov, A.K. Geim, S.V. Morozov et al., Two-dimensional gas of massless dirac fermions in graphene. Nature 438, 197–200 (2005). https://doi.org/10.1038/nature04233
K. Nordlund, J. Keinonen, T. Mattila, Formation of ion irradiation induced small-scale defects on graphite surfaces. Phys. Rev. Lett. 77, 699–702 (1996). https://doi.org/10.1103/PhysRevLett.77.699
A. Hashimoto, K. Suenaga, A. Gloter et al., Direct evidence for atomic defects in graphene layers. Nature 430, 870–873 (2004). https://doi.org/10.1038/nature02817
P.O. Lehtinen, A.S. Foster, Y. Ma et al., Irradiation-induced magnetism in graphite: a density functional study. Phys. Rev. Lett. 93, 187202 (2004). https://doi.org/10.1103/PhysRevLett.93.187202
O.V. Yazyev, L. Helm, Defect-induced magnetism in graphene. Phys. Rev. B 7, 125408 (2007). https://doi.org/10.1103/PhysRevB.75.125408
V.M. Pereira, D.S. Lopes, N.A. Castro, Modeling disorder in graphene. Phys. Rev. B 77, 115109 (2008). https://doi.org/10.1103/PhysRevB.77.115109
O.V. Yazyev, Magnetism in disordered graphene and irradiated graphite. Phys. Rev. Lett. 101, 037203 (2008). https://doi.org/10.1103/PhysRevLett.101.037203
J.C. Meyer, C. Kisielowski, R. Erni et al., Direct imaging of lattice atoms and topological defects in graphene membranes. Nano Lett. 8, 3582–3586 (2008). https://doi.org/10.1021/nl801386m
M.M. Ugeda, I. Brihuega, F. Guinea et al., Missing atom as a source of carbon magnetism. Phys. Rev. Lett. 104, 096804 (2010). https://doi.org/10.1103/PhysRevLett.104.096804
T. Kondo, Y. Honma, J. Oh et al., Edge states propagating from a defect of graphite: scanning tunneling spectroscopy measurements. Phys. Rev. B 82, 153414 (2010). https://doi.org/10.1103/PhysRevB.82.153414
B. Wen, M.S. Cao, M.M. Lu et al., Reduced graphene oxides: light-weight and high-efficiency electromagnetic interference shielding at elevated temperatures. Adv. Mater. 26, 3484–3489 (2014). https://doi.org/10.1002/adma.201400108
M.S. Cao, X.X. Wang, W.Q. Cao et al., Thermally Driven Transport and Relaxation Switching Self-Powered Electromagnetic Energy Conversion. Small 14, 1800987–1800994 (2018). https://doi.org/10.1002/smll.201800987
W.Q. Cao, X.X. Wang, J. Yuan et al., Temperature dependent microwave absorption of ultrathin graphene composites. J. Mater. Chem. C 3, 10017–10022 (2015). https://doi.org/10.1039/c5tc02185e
W.L. Song, M.S. Cao, Z.L. Hou et al., High dielectric loss and its monotonic dependence of conducting-dominated multiwalled carbon nanotubes/silica nanocomposite on temperature ranging from 373 to 873 K in X-band. Appl. Phys. Lett. 94, 233110 (2009). https://doi.org/10.1063/1.3152764
Y.N. Tang, Z.Y. Liu, Z.G. Shen et al., Adsorption sensitivity of metal atom decorated bilayer graphene toward toxic gas molecules (CO, NO, SO2 and HCN). Sensor. Actuat. B Chem. 238, 182–195 (2017). https://doi.org/10.1016/j.snb.2016.07.039
Y.N. Tang, H.D. Chai, W.G. Chen et al., Theoretical study on geometric, electronic and catalytic performances of Fe dopant pairs in graphene. Phys. Chem. Chem. Phys. 19, 26369–26380 (2017). https://doi.org/10.1039/c7cp05683d
Y.N. Tang, W.G. Chen, Z.G. Shen et al., Nitrogen coordinated silicon-doped graphene as a potential alternative metal-free catalyst for CO oxidation. Carbon 111, 448–458 (2017). https://doi.org/10.1016/j.carbon.2016.10.028
Y.N. Tang, H.D. Chai, H.W. Zhang et al., Tuning the adsorption and interaction of CO and O-2 on graphene-like BC3-supported non-noble metal atoms. Phys. Chem. Chem. Phys. 20, 14040–14052 (2018). https://doi.org/10.1039/c8cp00772a
Y.N. Tang, Z.G. Shen, Y.Q. Ma et al., Divacancy-nitrogen/boron-codoped graphene as a metal-free catalyst for high-efficient CO oxidation. Mater. Chem. Phys. 207, 11–22 (2018). https://doi.org/10.1016/j.matchemphys.2017.12.048
L.H. Yao, W.Q. Cao, M.S. Cao et al., Do** effect on the adsorption of Na atom onto graphenes. Curr. Appl. Phys. 16, 574–580 (2016). https://doi.org/10.1016/j.cap.2016.03.001
C. Marina, C. Simone, F.T. Gian et al., Structure and stability of hydrogenated carbon atom vacancies in graphene. Carbon 77, 165–174 (2014). https://doi.org/10.1016/j.carbon.2014.05.018
K. Yamashita, M. Saito, T. Oda, Atomic geometry and stability of mono-, di-, and trivacancies in graphene. Jpn. J. Appl. Phys. 45, 6534–6536 (2006). https://doi.org/10.1143/JJAP.45.6534
A.A. El-Barbary, R.H. Telling, C.P. Ewels et al., Structure and energetics of the vacancy in graphite. Phys. Rev. B 68, 144107 (2003). https://doi.org/10.1103/PhysRevB.68.144107
A.V. Krasheninnikov, P.O. Lehtinen, A.S. Foster et al., Bending the rules: contrasting vacancy energetics and migration in graphite and carbon nanotubes. Chem. Phys. Lett. 418, 132–147 (2006). https://doi.org/10.1016/j.cplett.2005.10.106
D.Q. **a, C.L. Ren, W. Zhang et al., Theoretical study of the interaction between metallic fission products and defective graphite. Comput. Mater. Sci. 106, 129–134 (2015). https://doi.org/10.1016/j.commatsci.2015.04.029
Y. Lei, A.S. Stephen, W.G. Zhu et al., Hydrogen-induced magnetization and tunable hydrogen storage in graphitic structures. Phys. Rev. B 77, 134114 (2008). https://doi.org/10.1103/PhysRevB.77.134114
Q.G. Jiang, Z.M. Ao, W.T. Zheng et al., Enhanced hydrogen sensing properties of graphene by introducing a mono-atom-vacancy. Phys. Chem. Chem. Phys. 15, 21016–21022 (2013). https://doi.org/10.1039/c3cp52976b
G.K. Sunnardianto, I. Maruyama, K. Kusakabe, Dissociation-chemisorption pathways of H2 molecule on graphene activated by a hydrogenated mono-vacancy V11. Adv. Sci. Eng. Med. 8, 421–426 (2016). https://doi.org/10.1166/asem.2016.1875
G.K. Sunnardianto, I. Maruyama, K. Kusakabe, Storing-hydrogen processes on graphene activated by atomic-vacancies. Int. J. Hydrogen Energy 42, 23691–23697 (2017). https://doi.org/10.1016/j.ijhydene.2017.01.115
P. Hohenberg, W. Kohn, Inhomogeneous electron gas. Phys. Rev. B 136, 864 (1964). https://doi.org/10.1103/PhysRev.136.B864
W. Kohn, L.J. Sham, Self-consistent equations including exchange and correlation effects. Phys. Rev. B 140, 1133 (1965). https://doi.org/10.1103/PhysRev.140.A1133
G. Kresse, J. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). https://doi.org/10.1103/PhysRevB.54.11169
J.P. Perdew, J.A. Chevary, S.H. Vosko et al., Atoms, molecules, solids, and surfaces-applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992). https://doi.org/10.1103/PhysRevB.46.6671
P.E. Blochl, Projector Augmented-Wave Method. Phys. Rev. B 50, 17953–17979 (1994). https://doi.org/10.1103/PhysRevB.50.17953
G. Henkelman, B.P. Uberuaga, H. Jonsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000). https://doi.org/10.1063/1.1329672
G. Henkelman, H. Jonsson, Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000). https://doi.org/10.1063/1.1323224
C. Li, C. Fang, C. Yang, First-principle studies of radioactive fission productions Cs/Sr/Ag/I adsorption on chrome–molybdenum steel in Chinese 200 MW HTR-PM. Nucl. Sci. Technol. 28, 79–88 (2017). https://doi.org/10.1007/s41365-017-0241-8
V. Morón, P. Gamallo, R. Sayós, DFT and kinetics study of O/O2 mixturesreacting over a graphite (0001) basal surface. Theor. Chem. Acc. 128, 683–694 (2011). https://doi.org/10.1007/s00214-010-0798-3
J. Fayos, Possible 3D carbon structures as progressive intermediates ingraphite to diamond phase transition. J. Solid State Chem. 148, 278–285 (1999). https://doi.org/10.1006/jssc.1999.8448
X.W. Sha, B. Jackson, First-principles study of the structural and energetic properties of H atoms on a graphite (0001) surface. Surf. Sci. 496, 318–330 (2002). https://doi.org/10.1016/S0039-6028(01)01602-8
D.W. Boukhvalov, M.I. Katsnelson, A.I. Lichtenstein, Hydrogen on graphene: electronic structure, total energy, structural distortions and magnetism from first-principles calculations. Phys. Rev. B 77, 035427 (2008). https://doi.org/10.1103/PhysRevB.77.035427
Y. Miura, H. Kasai, W. Dino et al., First principles studies for the disociative adsorption of H2 on graphene. J. Appl. Phys. 93, 3395–3400 (2003). https://doi.org/10.1063/1.1555701
P.O. Lethinen, A.S. Foster, Y. Ma et al., Irradiation induced magnetism in graphene: a density functional study. Phys. Rev. Lett. 93, 187202 (2004). https://doi.org/10.1103/PhysRevLett.93.187202
O.V. Yazyev, L. Helm, Defect induced magnetism in graphene. Phys. Rev. B 75, 125408 (2007). https://doi.org/10.1103/PhysRevB.75.125408
M.W.C. Dharma-Wardana, M.Z. Zgierski, Magnetism and structure at vacant lattice sites in graphene. Phys. E 41, 80–83 (2008). https://doi.org/10.1016/j.physe.2008.06.007
X.Q. Dai, J.H. Zhao, M.H. **e et al., First-principle study on magnetism induced by Vacanies in graphene. Eur. Phys. J. B 80, 343–351 (2011). https://doi.org/10.1140/epjb/e2011-10955-x
F. Banhart, J. Kotakoski, A.V. Krasheninnikov, Structural Defects in Graphene. ACS Nano 5, 26–41 (2011). https://doi.org/10.1021/nn102598m
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work is supported by the National Natural Science Foundation of China (Grant No. 51601212; 11475082) and “Strategic Priority Research Program of Chinese Academy of Sciences” Thorium Molten Salts Reactor Fund.
Rights and permissions
About this article
Cite this article
Wu, XJ., Fei, ZJ., Liu, WG. et al. Adsorption and desorption of hydrogen on/from single-vacancy and double-vacancy graphenes. NUCL SCI TECH 30, 69 (2019). https://doi.org/10.1007/s41365-019-0584-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-019-0584-4