Abstract
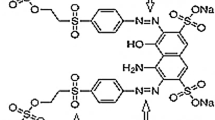
Azo dyes have great application in industries like textile. However, their presence poses a lot of concerns to the environment and human health such as carcinogenic effect. Electrochemical processes such as peroxi-coagulation (PC) are cost effective and efficient advanced oxidation processes which have been investigated extensively. This study aimed to conduct a comparative study on removal of Acid red 131 as an azo dye via PC process using stainless steel (SS) and aluminum (Al) electrodes as anode and graphite as cathode. Parameters including dye concentration, current density, initial pH, aeration rate, and electrode’s surface area were investigated. According to the results, the optimum condition for both electrodes was achieved at electrode’s surface area = 60 cm2, pH = 7, and aeration rate = 1.5 L/min. Also, the optimum current of 0.6 A and 0.9 A were selected for SS and Al, respectively. The removal percentages at these conditions were measured 98% and 93% after 120 min for SS and Al, respectively. Chemical oxygen demand (COD) removal was also investigated, and the removal percentage was recorded 93% and 79% for SS and Al after 180 min, respectively. The removal kinetics studies indicated that the pseudo-first order model best fitted for both electrodes. Based on the results, the SS electrode outperformed the Al electrode and facilitated the process.











Similar content being viewed by others

Data Availability
The data are available upon request to the corresponding author.
Code Availability
Not applicable.
References
Rahmati R, Nayebi B, Ayati B (2021) Investigating the effect of hydrogen peroxide as an electron acceptor in increasing the capability of slurry photocatalytic process in dye removal. Water Sci Technol. https://doi.org/10.2166/wst.2021.136
Ferrero F, Periolatto M (2012) Functionalized fibrous materials for the removal of dyes. Clean Technol Environ Policy 14:487–494. https://doi.org/10.1007/s10098-011-0442-5
J Perera à (2019) Removal of acid orange 7 dye from wastewater: review. Int J Waste Resour 09. https://doi.org/10.35248/2252-5211.19.9.367
Brillas E, Garcia-Segura S (2016) Solar photoelectro-fenton degradation of acid orange 7 azo dye in a solar flow plant: optimization by response surface methodology. Water Conserv Sci Eng 1:83–94. https://doi.org/10.1007/s41101-016-0005-z
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971. https://doi.org/10.1016/j.envint.2004.02.001
Ghalebizade M, Ayati B (2019) Acid Orange 7 treatment and fate by electro-peroxone process using novel electrode arrangement. Chemosphere 235:1007–1014. https://doi.org/10.1016/j.chemosphere.2019.06.211
Khandegar V, AnilK S (2014) Electrochemical treatment of textile effluent containing acid red 131 dye. J Hazard Toxic Radioact Waste 18:38–44. https://doi.org/10.1061/(asce)hz.2153-5515.0000194
Fernandes NC, Brito LB, Costa GG et al (2018) Removal of azo dye using Fenton and Fenton-like processes: evaluation of process factors by Box-Behnken design and ecotoxicity tests. Chem Biol Interact 291:47–54. https://doi.org/10.1016/J.CBI.2018.06.003
Bahadori E, Rapf M, di Michele A, Rossetti I (2020) Photochemical vs. photocatalytic azo-dye removal in a pilot free-surface reactor: is the catalyst effective? Sep Purif Technol 237:116320. https://doi.org/10.1016/J.SEPPUR.2019.116320
Le Huong TX, Alemán B, Vilatela JJ et al (2018) Enhanced electro-fenton mineralization of acid orange 7 using a carbon nanotube fiber-based cathode. Front Mater 5. https://doi.org/10.3389/fmats.2018.00009
dos Santos AJ, Fajardo AS, Kronka MS et al (2021) Effect of electrochemically-driven technologies on the treatment of endocrine disruptors in synthetic and real urban wastewater. Electrochim Acta 376:138034. https://doi.org/10.1016/J.ELECTACTA.2021.138034
de Luna MDG, Gumaling RP, Barte EG et al (2021) Electrochemically-driven regeneration of iron (II) enhances Fenton abatement of pesticide cartap. J Hazard Mater 126713. https://doi.org/10.1016/J.JHAZMAT.2021.126713
Panizza M, Cerisola G (2009) Direct And Mediated Anodic Oxidation of Organic Pollutants. Chem Rev 109:6541–6569. https://doi.org/10.1021/cr9001319
Garcia-Segura S, Lima ÁS, Cavalcanti EB, Brillas E (2016) Anodic oxidation, electro-Fenton and photoelectro-Fenton degradations of pyridinium- and imidazolium-based ionic liquids in waters using a BDD/air-diffusion cell. Electrochim Acta 198:268–279. https://doi.org/10.1016/J.ELECTACTA.2016.03.057
Davarnejad R, Sabzehei M, Parvizi F et al (2019) Study on soybean oil plant wastewater treatment using the electro-fenton technique. Chem Eng Technol 42:2717–2725. https://doi.org/10.1002/ceat.201800765
Mohajeri S, Hamidi AA, Isa MH, Zahed MA (2019) Landfill leachate treatment through electro-fenton oxidation. Pollution 5:199–209. https://doi.org/10.22059/poll.2018.249210.364
Dehboudeh M, Dehghan P, Azari A, Abbasi M (2020) Experimental investigation of petrochemical industrial wastewater treatment by a combination of integrated fixed-film activated sludge (IFAS) and electro-Fenton methods. J Environ Chem Eng 8. https://doi.org/10.1016/j.jece.2020.104537
Nidheesh PV, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15. https://doi.org/10.1016/j.desal.2012.05.011
Huo S, Necas D, Zhu F et al (2021) Anaerobic digestion wastewater decolorization by H2O2-enhanced electro-Fenton coagulation following nutrients recovery via acid tolerant and protein-rich Chlorella production. Chem Eng J 406. https://doi.org/10.1016/j.cej.2020.127160
Chu Y, Miao B, Zhang X, Lv R (2020) Heterogeneous electro-Fenton-like oxidation for the degradation of 4-nitrophenol characterized by immobilized Fe(III): performance, mechanism and chlorinated organic compounds formation. J Water Process Eng 38. https://doi.org/10.1016/j.jwpe.2020.101662
Can OT (2014) COD removal from fruit-juice production wastewater by electrooxidation electrocoagulation and electro-Fenton processes. Desalin Water Treat 52:65–73. https://doi.org/10.1080/19443994.2013.781545
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B 166–167:603–643. https://doi.org/10.1016/j.apcatb.2014.11.016
Adimi M, Mohammad Mohebizadeh S, Poor MM et al (2019) Treatment of Shazand Petrochemical Co., Effluent using electro-fenton method modified with iron nanoparticles and anodic aluminum oxide electrode: a comparison. Iran J Sci Technol Trans A Sci 43:2799–2806. https://doi.org/10.1007/s40995-019-00766-6
Yavuz Y, Shahbazi R, Koparal AS, Öǧütveren ÜB (2014) Treatment of Basic Red 29 dye solution using iron-aluminum electrode pairs by electrocoagulation and electro-Fenton methods. Environ Sci Pollut Res 21:8603–8609. https://doi.org/10.1007/s11356-014-2789-8
Nidheesh, PV (2018) Removal of organic pollutants by peroxicoagulation. Environ Chem Lett 16:1283–1292. https://doi.org/10.1007/s10311-018-0752-5
Ren G, Zhou M, Su P et al (2018) Highly energy-efficient removal of acrylonitrile by peroxi-coagulation with modified graphite felt cathode: Influence factors, possible mechanism. Chem Eng J 343:467–476. https://doi.org/10.1016/J.CEJ.2018.02.115
Garcia-Segura S, Eiband MMSG, de Melo JV, Martínez-Huitle CA (2017) Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J Electroanal Chem 801:267–299
Brillas E, Casado J (2002) Aniline degradation by Electro-Fenton® and peroxi-coagulation processes using a flow reactor for wastewater treatment. Chemosphere 47:241–248. https://doi.org/10.1016/S0045-6535(01)00221-1
Zarei M, Salari D, Niaei A, Khataee A (2009) Peroxi-coagulation degradation of C.I. Basic Yellow 2 based on carbon-PTFE and carbon nanotube-PTFE electrodes as cathode. Electrochim Acta 54:6651–6660. https://doi.org/10.1016/j.electacta.2009.06.060
do Vale-Júnior E, da Silva DR, Fajardo AS, Martínez-Huitle CA (2018) Treatment of an azo dye effluent by peroxi-coagulation and its comparison to traditional electrochemical advanced processes. Chemosphere 204:548–555. https://doi.org/10.1016/j.chemosphere.2018.04.007
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227. https://doi.org/10.1016/J.CHEMOSPHERE.2017.12.195
Association APH, Association AWW, Federation WE (2017) Standard methods for the examination of water and wastewater. American Public Health Association
Phetrak A, Westerhoff P, Garcia-Segura S (2020) Low energy electrochemical oxidation efficiently oxidizes a common textile dye used in Thailand. J Electroanal Chem 871:114301. https://doi.org/10.1016/J.JELECHEM.2020.114301
Stupar SL, Grgur BN, Radišić MM et al (2020) Oxidative degradation of Acid Blue 111 by electro-assisted Fenton process. J Water Process Eng 36. https://doi.org/10.1016/j.jwpe.2020.101394
Zarei M, Niaei A, Salari D, Khataee AR (2010) Removal of four dyes from aqueous medium by the peroxi-coagulation method using carbon nanotube–PTFE cathode and neural network modeling. J Electroanal Chem 639:167–174. https://doi.org/10.1016/J.JELECHEM.2009.12.005
Palanisamy S, Nachimuthu P, Awasthi MK et al (2020) Application of electrochemical treatment for the removal of triazine dye using aluminium electrodes. J Water Supply Res Technol AQUA 69:345–354. https://doi.org/10.2166/aqua.2020.109
Salari D, Niaei A, Khataee A, Zarei M (2009) Electrochemical treatment of dye solution containing CI Basic Yellow 2 by the peroxi-coagulation method and modeling of experimental results by artificial neural networks. J Electroanal Chem 629:117–125
Brillas E, Sauleda R, Casado J (1998) Degradation of 4-chlorophenol by anodic oxidation, electro-fenton, photoelectro-fenton, and peroxi-coagulation processes. J Electrochem Soc 145:759–765. https://doi.org/10.1149/1.1838342
Boye B, Dieng MM, Brillas E (2003) Electrochemical degradation of 2,4,5-trichlorophenoxyacetic acid in aqueous medium by peroxi-coagulation. Effect of pH and UV light. Electrochim Acta 48:781–790. https://doi.org/10.1016/S0013-4686(02)00747-8
Ghanbari F, Moradi M (2015) A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: electrical energy consumption and biodegradability improvement. J Environ Chem Eng 3:499–506. https://doi.org/10.1016/j.jece.2014.12.018
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B 87:105–145. https://doi.org/10.1016/j.apcatb.2008.09.017
Khataee AR, Safarpour M, Zarei M, Aber S (2011) Electrochemical generation of H2O2 using immobilized carbon nanotubes on graphite electrode fed with air: Investigation of operational parameters. J Electroanal Chem 659:63–68. https://doi.org/10.1016/j.jelechem.2011.05.002
Khandegar V, K SAnil, (2014) Electrochemical treatment of textile effluent containing acid red 131 dye. J Hazard Toxic Radioact Waste 18:38–44. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000194
Daneshvar N, Khataee AR, Amani Ghadim AR, Rasoulifard MH (2007) Decolorization of C.I. Acid Yellow 23 solution by electrocoagulation process: Investigation of operational parameters and evaluation of specific electrical energy consumption (SEEC). J Hazard Mater 148:566–572. https://doi.org/10.1016/J.JHAZMAT.2007.03.028
Brillas E, Sirés I, Cabot PL (2010) Use of both anode and cathode reactions in wastewater treatment. In: Comninellis C, Chen G (eds) Electrochemistry for the environment. Springer, New York, pp 515–552
Yavuz Y, Öcal E, Koparal AS, Öğütveren ÜB (2011) Treatment of dairy industry wastewater by EC and EF processes using hybrid Fe• Al plate electrodes. J Chem Technol Biotechnol 86:964–969. https://doi.org/10.1002/jctb.2607
Varank G, Yazici Guvenc S, Demir A (2018) A comparative study of electrocoagulation and electro-Fenton for food industry wastewater treatment: Multiple response optimization and cost analysis. Sep Sci Technol 53:2727–2740. https://doi.org/10.1080/01496395.2018.1470643
Nayebi B, Ayati B (2021) Degradation of emerging amoxicillin compound from water using the electro-fenton process with an aluminum anode. Water Conserv Sci Eng. https://doi.org/10.1007/s41101-021-00101-4
Malakootian M, Moridi A (2017) Efficiency of electro-Fenton process in removing Acid Red 18 dye from aqueous solutions. Process Saf Environ Prot 111:138–147. https://doi.org/10.1016/j.psep.2017.06.008
Benitez FJ, Real FJ, Acero JL et al (2007) Kinetics of phenylurea herbicides oxidation by Fenton and photo-Fenton processes. J Chem Technol Biotechnol 82:65–73. https://doi.org/10.1002/jctb.1638
Yazdanbakhsh AR, Massoudinegad MR, Eliasi S, Mohammadi AS (2015) The influence of operational parameters on reduce of azithromyin COD from wastewater using the peroxi-electrocoagulation process. Journal of Water Process Engineering 6:51–57. https://doi.org/10.1016/j.jwpe.2015.03.005
Devika V, Gandhimathi R, Nidheesh PV, Ramesh ST (2016) Effect of solution pH on leachate treatment mechanism of peroxicoagulation process. J Hazard Toxic Radioact Waste 20:06016001. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000315
Kumar A, Nidheesh PV, Suresh Kumar M (2018) Composite wastewater treatment by aerated electrocoagulation and modified peroxi-coagulation processes. Chemosphere 205:587–593. https://doi.org/10.1016/J.CHEMOSPHERE.2018.04.141
Yıldıza N, Gökkuşa Ö, Koparalb AS, Yıldıza YŞ (2019) Peroxi-coagulation process: a comparison of the effect of oxygen level on color and TOC removals. Desalin Water Treat 1:9
Kishi A, Inoue M, Umeda M (2013) Evaluation of H2O2-generation during oxygen reduction at electrodeposited Pt particles on mask scratched electrodes. Appl Surf Sci 279:245–249. https://doi.org/10.1016/j.apsusc.2013.04.074
Guivarch E, Trevin S, Lahitte C, Oturan MA (2003) Degradation of azo dyes in water by Electro-Fenton process. Environ Chem Lett 1:38–44. https://doi.org/10.1007/s10311-002-0017-0
Nayir TY, Dinc O, Kara S et al (2020) Laundry wastewater treatment by peroxi-coagulation. Desalin Water Treat 182:98–108. https://doi.org/10.5004/dwt.2020.25188
Brillas E, Boye B, Dieng MM (2003) Peroxi-coagulation and photoperoxi-coagulation treatments of the herbicide 4-chlorophenoxyacetic acid in aqueous medium using an oxygen-diffusion cathode. J Electrochem Soc 150:E148. https://doi.org/10.1149/1.1543950
Sandhwar VK, Prasad B (2018) Comparison of electrocoagulation, peroxi-electrocoagulation and peroxi-coagulation processes for treatment of simulated purified terephthalic acid wastewater: optimization, sludge and kinetic analysis. Korean J Chem Eng 35:909–921. https://doi.org/10.1007/s11814-017-0336-2
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Behnam Nayebi and Mohammad Ghalebizade. The first draft of the manuscript was written by Kasra Pourrostami Niavol and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nayebi, B., Ghalebizade, M. & Niavol, K.P. Removal of Acid Red 131 by Peroxi-Coagulation Using Stainless Steel and Aluminum Electrodes: a Comparative Study. Water Conserv Sci Eng 6, 201–211 (2021). https://doi.org/10.1007/s41101-021-00114-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-021-00114-z