Abstract
In this study, the stress–strain curves of sandstone soaked in 5% NaCl and 5% K2SO4 solutions under different loading and unloading paths were obtained by carrying out different paths of unloading confining pressure tests on sandstone specimens. Thereafter, the strength, deformation, and failure characteristics of the specimens were analysed. First, the unloading capacity of sandstone specimens soaked in 5% NaCl was found to be greater than those soaked in 5% K2SO4, and the unloading amount increased in the order of increasing axial pressure unloading confining pressure < constant axial pressure unloading confining pressure < constant deviatoric stress unloading confining pressure. When unloading the confining pressure, the strain change rate of the sandstone specimens soaked in 5% K2SO4 was greater than that of the sandstone specimen soaked in 5% NaCl, and the strain change rate had the following trend: increasing adding axial pressure and unloading confining pressure > constant axial pressure unloading confining pressure > constant deviatoric stress unloading confining pressure. The deformation modulus and Poisson's ratio of the sandstone specimens during unloading exhibited an exponential change with increasing unloading ratio. The deformation modulus and Poisson’s ratio of the sandstone soaked in 5% NaCl during the unloading period was respectively greater and less than those of the sandstone soaked in 5% K2SO4. Second, the total energy absorbed by 5% NaCl soaked sandstone specimens was always less than that soaked in 5% K2SO4 under different unloading paths. The effect of immersing the specimens in different solution on acoustic emission and failure characteristics was unclear. Compared with the dry sandstone specimens, the sandstone specimens soaked in brine solutions lower mineral composition and higher, clay mineral content. The contents of plagioclase and calcite contents in the sandstone specimens soaked in 5% NaCl was similar to those in dry sandstone, but no plagioclase or calcite was detected in the specimens soaked in 5% K2SO4.
Article Highlights
-
Investigate the strength and deformation failure behavior of sandstones immersed in 5% NaCl and 5% K2SO4.
-
Analyze the energy evolution of brined sandstone under different confining pressure unloading paths.
-
Explore the microstructure characteristics of brined sandstone under different confining pressure unloading paths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The interaction between groundwater and rocks is gaining increasing attention in geotechnical engineering. Groundwater containing various of ions affects rock mechanically, physically and chemically (Wang et al. 2017; Han et al. 2013), thereby causing changes in the internal composition and structure of rocks. This phenomenon results in variations in the mechanical properties of rocks and affects the safety of projects. Moreover, during excavation processes, the initial stress conditions of rocks are disrupted and the internal stresses are redistributed to attain the corresponding new equilibrium, and engineering disasters often occur during the stress rebalancing process (Wang et al. 2007). Further, the impact of excavation unloading on the stability of geotechnical engineering is particularly prominent in groundwater-rich areas. A comprehensive consideration of the influence of the water chemistry environment and stress variation on the mechanical properties of rocks is an important will be a guideline for engineering construction in disturbed aquatic zones.
Scrivano and Gaggero (2020) studied the corrosivity of building limestone with different porosities in saturated brine solutions (Na2SO4, MgSO4, Na2CO3, NaCl, and KNO3) and found that saturated Na2SO4 and Na2CO3 solutions were more corrosive than other saturated brine solutions. Rathnaweera et al. (20142015) conducted the mechanical test on sandstone soaked in different brine concentrations (0, 10, 20 and 30%) and found that the uniaxial compressive strength of 10% brine saturated sandstone specimens were lower than that water saturated sandstone specimens, where 20% and 30% brine saturated sandstone specimens were higher. Haider et al. (2013) studied the uniaxial compression mechanical properties of ordinary Portland cement and geopolymer cement after immersion in saline solution of different concentrations (0, 8 and 15%) and found that geopolymer cement in saline solution has higher strength than ordinary Portland cement. Nasvi et al. (2014) soaked well sealing materials and sandstone in NaCl solutions of different concentrations (0, 5 and 15%) for different duration (0, 30, 60 and 90 d), and then conducted uniaxial compression tests to explore the relationship among their mechanical parameters, brine concentration and soaking time.
Previous studies have reported that the mechanical properties of rocks in the unloading condition are completely different from those in the conventional compression condition. Li (2010) investigated the stress–strain and damage characteristics of sandstone under unloading stress conditions using triaxial unloading damage experiments. The unloading quantity was regarded as an important parameter for deriving the elastic-brittle plasticity mechanical model considering the unloading stress condition. By conducting different unloading path tests on siltstone, Li et al. (2015) demonstrated that the unloading stress path has a significant influence on the primary mechanical parameters of rocks and confining pressure. Further, Li et al. (20172016a) compared the energy evolution of granite under two stress paths, that is increasing axial pressure unloading confining pressure and constant axial pressure unloading confining pressure, and found that the former was more prone to rock burst. They also found that the controlling parameters for rock failure under these two stress paths are cohesion and friction. Zhao et al. (2015) reported that the initial confining pressure has a significantly higher effect on the strain energy than the unloading path, and both the unloading path and initial confining pressure have substantial impacts on energy dissipation.
In recent years, the unloading mechanical properties of rocks in groundwater environments have been investigated. Using rock mechanics experiments, Hu (2016) found that sandstone is damaged and its brittleness is decreased as water–rock action intensifies. By conducting constant axial pressure unloading tests on dry and water-saturated soft sandstone, Li et al. (2016b) discovered that the cohesion and internal friction angle of sandstone respectively increase and decrease during unloading compared with the loaded condition in both dry and water-saturated conditions, and these variations were considerably higher in the water-saturated rocks than in the dry rock. However, previous unloading tests have usually been conducted on dry rocks, and there are few studies on water-saturated rock specimens, and even fewer investigations on unloading mechanics using brine as the rock saturation solution.
To study the stability of CO2 storage in deep brine in the Sichuan Basin, sandstone specimens were soaked according to the characteristics of the brine in the basin. Saline layers in Sichuan Basin contain Na+, K+, Mg2+, Ca2+, Cl−, SO 2−4 and HCO−3 ions, and Na+, K+, Cl− and SO 2−4 ions account for approximately 90% of the total ions on average. The average salinity of brine is 285.5 g/L, the highest concentration of NaCl ion is 23% (g/g), and the highest concentration of K2SO4 ions is 5.18% (g/g) (Lin et al. 2002a). In this study, unloading mechanical experiments were performed to study the influence of two types of saline solutions (5% NaCl and 5% K2SO4) on mechanical, acoustic, and fracture behaviors of sandstone specimens during different unloading paths. Additionally, this work analyzed the microscopic characteristics of rocks under different immersion solutions to further elucidate the influence of the chemical solutions on the mechanical behaviour of sandstone specimens.
2 Experimental material and procedure
2.1 Specimen preparation
The sandstone was obtained from Zigong City, Sichuan Province, and standard cylindrical of 50 mm and height of 100 mm were machined from this rock using an apparatus. The uniaxial compression test results of standard sandstone specimens are listed in Table 1. The average peak strength, average elastic modulus, and average deformation modulus of the sandstone specimens were 93.01 MPa, 11.84 GPa and 8.05 GPa, respectively. Discretization analysis revealed, the uniaxial compressive strengths of the specimens were similar, which indicated that the rocks were highly homogeneous (Yang et al. 2021). The main ions in the brine layer of the Sichuan Basin are Na+, K+, Cl− and SO42−, with the concentrations of NaCl and K2SO4 reaching 23% (g/g) and 5.18% (g/g), respectively (Lin et al. 2002b). Thus, in the present study, 5% NaCl and 5% K2SO4 solutions were prepared according to methods of a previous study. Subsequently, the specimens were placed in containers with each of the prepared solutions and immersed in vacuum for 2 months, as shown in Fig. 1.
2.2 Experimental equipment and procedures
The unloading test in this study was conducted using the GCTS RTX-4000 testing system (Fig. 2) at the State Key Laboratory for Geomechanics and Deep Underground Engineering, China University of Mining and Technology (Yang et al. 2018). The test system can apply up to 140 MPa of confining pressure and up to 4000 kN of axial pressure, with a range of ± 2.5 mm for the axial and circumferential displacement transducers.
In this study, four stress paths were used to conduct the triaxial tests on sandstone after chemical solution immersion (Fig. 3). The specific experimental procedures are presented as follows.
• Stress paths I: Conventional triaxial test
Hydrostatic pressure was applied to a specimen at a rate of 5 MPa/min until the desired confining pressure (σ3 = 39 MPa) was reached. Next, the confining pressure was maintained, and the specimen was compressed at an axial displacement rate of 0.06%/min until it failed.
• Stress paths II: Increasing axial pressure and unloading confining pressure test
The path simulated the stress redistribution procedure of increasing tangential stress and decreasing radial stress during the excavation of rocks from deep saline layer. The loading rate and mode of the confining pressure and axial pressure were the same as those of the conventional triaxial test. When the axial pressure was loaded to 80% of the triaxial compressive strength, unloading of the confining pressure started, at an unloading rate of 0.04 MPa/s, whereas the axial pressure was loaded at a rate of 0.06%/min.
• Stress paths III: Constant axial pressure and unloading confining pressure test
The path simulated the stress redistribution procedure of a constant tangential stress and decreased radial stress during rock excavation in a deep saline layer. The loading rate and approach of the confining and axial pressure stages were the same as those of the conventional triaxial test. When the axial pressure was loaded to 80% of the triaxial compressive strength, the axial pressure was constant and the confining pressure was unloaded at a rate of 0.04 MPa/s.
• Stress paths IV: Constant deviatoric stress and unloading confining pressure test
This path simulated the stress redistribution process of simultaneously decreasing tangential stress and radial stress during rock excavation in deep saline layer. The loading rates and approaches of the confining and axial pressure stages were the same as those of the conventional triaxial test, and the axial pressure and confining pressure were unloaded at a rate of 0.04 MPa/s when the axial pressure was loaded to 80% of the triaxial compressive strength.
Table 2 list the physical parameters and experimental conditions for the sandstone specimens used in this study.
3 Strength and deformation behaviors
3.1 Stress–strain curves
Figures 4 and 5 illustrate the triaxial unloading stress–strain curves of the sandstone specimens soaked in different saline solutions, demonstrating that the stress–strain curves under unloading confining pressure and the conventional triaxial stress–strain curve are different, which is mainly portrayed as changes in the variation patterns of axial strain and hoop strain following unloading confining pressure as well as unloading paths.
The stress–strain curve of the conventional triaxial test mainly includes the elastic, yield, and failure stages. Before unloading, the stress–strain curves under different unloading paths in this study were similar to the stress–strain curve of the conventional triaxial test. In contrast, the axial strain and hoop strain of the specimens increase continuously on the heels of unloading, with distinct expansion characteristics. The axial strain of the specimens begins to change nonlinearly and yield with increasing stress after unloading in stress path II. Simultaneously, the circumferential deformation grows rapidly, and the volume deformation increase inversely. Both the decrease in confining pressure and increase in axial compression lead to the brittle failure of the specimens. At the beginning of unloading in stress path III, the axial stress remains unchanged, loop stress gradually declines, stress difference gradually increases, yield occurs quickly, and growth of circumferential deformation steepens. Meanwhile, stress path IV unloaded the axial compression and confining pressure at the same rate, which guaranteed the stability of the principal stress difference sustained by the specimens. The unloading process was characterized by unchanged deviatoric stress, continuous growth in axial strain and hoop strain, and an inverse increase in volume strain, thereby resulting in expansion deformation. The specimens reach the ultimate bearing capacity and suffer brittle failure when the confining pressure is unloaded to a certain extent. The stress-volume strain reveals that the deformation under stress path II is less than those under stress path III, as well as stress path IV.
3.2 Confining pressure-strain curves
The confining pressure and strain curve in the process of unloading the confining pressure was obtained to reveal the relationship between confining pressure and strain, as shown in Fig. 6. The figure confirms that strain varies with confining pressure under unloading paths. The axial strain changes linearly instead of instantaneously, which is attributed to the strain control employed to load the axial stress in stress path II. However, the hoop strain first dips linearly and then drops several times following the decrease in the confining pressure. The axial strain and hoop strain change linearly with the reduced confining pressure in the early stage of stress path III, followed by a nonlinear change with the further decrease in confining pressure. The loss of bearing capacity of the specimens results in a sudden strain change. The increase in the axial strain and hoop strain with decreasing confining pressure under stress path IV is in agreement with the outcome of stress path III. However, at the same unloading rate, the rate of change in the axial strain and hoop strain with decreasing confining pressure under stress path II is higher than that of stress path III, in addition to that of stress path IV, which can be explained by the growth of axial stress associated with the decline in loop stress in stress path II. The loop stress declines and the axial stress remains constant under stress path III, with a higher principal stress difference. However, both the axial stress and loop stress decrease with a stable principal stress difference under stress path IV. Hence, the rate of change in the axial and hoop strain under stress path III is higher than that under stress path IV.
Figure 7, shows the relationship between confining pressure and strain during unloading confining pressure of specimens soaked in saline solutions and reveals that, the unloading of specimen soaked in 5%NaCl outlasts that of specimens soaked in 5% K2SO4 under the same path. For the specimen soaked in 5% K2SO4, the unloading of a small amount of confining pressure is unloaded results in more failure. Additionally, the change in the strain of these specimens with decreasing confining pressure is greater than that of the specimens soaked in 5% NaCl, which is because of the cracks that appear between particles and a drop in tensile strength following the dissolution of plagioclase and calcite in the specimen soaked in K2SO4, as well as the high tensile strength of the specimens soaked in NaCl owing to its stable composition. Once the unloading of the confining pressure begins, the strain change rate of the specimens soaked in K2SO4 is higher than those soaked in NaCl owing to the greater weakening of the internal tensile capacity.
Table 3 presents the parameters of the specimens soaked in solutions under different unloading paths derived from the confining pressure unloading test, in which \(\vartriangle \varepsilon_{i}\) represents the variation in strain from the unloading to failure stage. The table reveals the following trends for the specimens soaked in the same solution, unloading values, stress path II < stress path III < stress path IV, change in axial strain stress path II < stress path IV < stress path III, change in loop strain stress path II < stress path III < stress path IV, and change in volume strain, stress path II < stress path III < stress path IV. The minimum changes of the axial, loop and volume strains are observed in stress path II owing to its higher axial pressure, faster failure, and confining pressure which barely decreases. The axial stress of the specimens remains unchanged in stress path III. However, the decrease in confining pressure and the increase in deviatoric stress, along with the decline in axial stress and loop stress of specimens in stress path IV, lead to less unloading and faster failure in stress path III than in stress path IV. Partial elastic rebound is observed in stress path IV because of the decline in axial stress, resulting in a smaller axial strain in stress path IV than in stress path III. However, the hoop strain and volume strain in stress path IV are higher than those in stress path III because of the larger unloading in stress path IV. The unloading amount and the strain change of the specimen soaked in 5% NaCl is respectively greater and less than those in 5% K2SO4 under stress path III. In contrast, specimens soaked in the two solutions under other paths experience negligible strain change during unloading confining pressure process, which can be explained by the sever erosion of the internal components of the specimen soaked in 5% K2SO4, resulting in lower tensile strength.
3.3 Analysis of confining pressure and deformation parameters
Despite the similarity between the loading of the unloading test and triaxial test, a major difference in strength and deformation was found. During unloading, the hoop strain gradually increases, and the internal pores gradually expands and evolves owing to the lower loop stress, resulting in deteriorating properties. The unloading ratio (Zhu et al. 2020), which consider the initial confining pressure and unloading amount, is introduced in this study to explore the evolution of the deformation parameters of the specimens during the unloading process, and its formula is depicted in Eq. 1.
where, H is the unloading ratio, \(\sigma_{3}^{{\text{T}}}\) is the real-time confining pressure, MPa; \(\sigma_{3}^{0}\) is the initial confining pressure of unloading, MPa.
The deformation parameters of the unloading test are required to account for the effects of loop stress and circumferential deformation given the difference between the unloading and triaxial tests. According to Hooke’s Law, the deformation modulus and Poisson’s ratio during the unloading test were calculated using Eq. 2 (Gao et al. 2005).
where E is the deformation modulus, GPa; \(\mu\) is Poisson's ratio; \(\sigma_{1}\) is the maximum principal stress, MPa; \(\sigma_{3}\) is the minimum principal stress, MPa; \(\varepsilon_{1}\) and \(\varepsilon_{3}\), are the axial and circumferential strains, respectively.
Figure 8a illustrates the variation curve of the deformation modulus of the specimens with the unloading ratio under different unloading paths. The deformation modulus change negligibly at the early unloading stage, and dips nonlinearly with further unloading of the confining pressure. Then it drops exponentially following the failure caused by the loss of bearing when the unloading ratio reaches a critical value. With a change in the unloading ratio, the deformation modulus of the specimen varies for different stress paths. The deformation modulus of the pre-failure specimen under stress path II undergoes nonlinear and noticeable change, followed by those under stress path III, and IV, which results from the axial stress in the process of unloading the confining pressure. The increase in axial stress incurs the sharpest drop in deformation modulus, the stability of axial stress incurs a medium drop, and the decline in axial stress incurs the least drop. The influence of soaking solutions on deformation modulus of specimens during unloading is mainly displayed as the greater deformation modulus of specimens soaked in 5% NaCl than those soaked in 5% K2SO4, indicating that the axial strain variation of specimens soaked in 5% K2SO4 peaks and more energy is absorbed in the process of unloading confining pressure, which explains its quicker failure than in specimens soaked in 5% NaCl.
Figure 8b displays the variation curve of Poisson’s ratio of specimens with unloading ratio during unloading under different unloading paths. The Poisson’s ratio of the specimens grows exponentially. In particular, it changes negligibly in the early unloading phase, grows rapidly with a further reduction of confining pressure, and soars with the loss of bearing capacity. The Poisson’s ratio of specimens soaked in 5% K2SO4 is higher than that of the specimens soaked in 5% NaCl in the unloading process under diverse unloading paths, indicating a higher sensitivity of circumferential deformation for the specimens soaked in K2SO4 to the confining pressure, and their inverse proportion.
3.4 Variation in dilatancy angle of sandstone under different unloading paths
To study the dilatancy characteristics of different immersed sandstone specimens under different unloading paths, the dilatancy angle was calculated in this study as shown in Eqs. 4 and 5.
where \(\Delta \varepsilon_{{\text{v}}}^{p}\) is the volume plastic strain increment; \(\Delta \varepsilon_{1}^{{\text{p}}}\) is the axial plastic strain increment; \(\varepsilon_{{\text{v}}}^{p}\) and \(\varepsilon_{{\text{v}}}^{e}\) are the volume plastic strain and volume elastic strain respectively; \(\varepsilon_{1}^{{\text{p}}}\) and \(\varepsilon_{1}^{{\text{e}}}\) are the axial plastic strain and volume elastic strain, respectively;
The variation in the sandstone dilatancy angle under different unloading paths are shown in Fig. 9. As shown in the figure, under the path of axial compression unloading confining pressure, the dilatancy angle of the sandstone specimens decreases nonlinearly with the unloading of confining pressure, the dilatancy angle decreases rapidly at the initial stage of the confining pressure reduction and then decreases gradually with further unloading of the confining pressure. This pattern is mainly because of the increase in axial stress in the process of unloading the confining pressure, which leads to an increase in the axial plastic strain of the specimen, thereby causing continuous reduction in the dilatancy angle of the specimen. The dilatancy angle first increases and then decreases under constant axial and deviatoric unloading confining pressures. Under these two paths, the circumferential strain energy is released when the confining pressure is discharged, and microcracks are generated inside the specimen. The plastic circumferential strain continues to increase, whereas the axial stress remains unchanged or decreases. The axial deformation does not respond to the process of confining pressure discharge, and the axial plasticity barely changes, thereby increasing the dilatancy angle. With further unloading of the confining pressure, the cracks inside the specimen evolve and penetrate, and the axial plastic strain increases, thereby leading to a decline in the dilatancy angle of the specimen.
4 Energy dissipation analysis
The testing machine was used for compressing the specimens. This process is assumed to be a closed system without any energy exchange with the outside environment (**e et al. 2005). According to the conservation of energy, the total work U exerted by the testing machine on the specimens should be consistent with Eq. 4. Figure 10 shows the schematic diagram of the strain–stress curves of the rocks, where \(U_{{\text{i}}}^{{\text{d}}}\) represents for the energy consumed, and the shaded area \(U_{{\text{i}}}^{e}\) is the releasable elastic strain energy stored in the rock. According to thermodynamics, energy dissipation is one-way and irreversible, whereas the energy release is two-way and conditionally reversible. The process of rock loading involves the initiation, development, and coalescence of internal cracks, forming macroscopic cracks, as well as energy input, storage, dissipation and release (Chen et al. 2016).
where \(U^{0}\) is the power generated by the testing machine on the specimen in the hydrostatic loading stage.
According to Fig. 11, which displays the energy variation curves of sandstone soaked in solutions under diverse unloading paths, the specimens under different paths exhibit similar trend in overall energy variation. In the elastic stage, the total energy and elastic energy curves overlap, and the total energy and elastic energy grow synchronously almost without dissipating their energy. A further increase in stress results in the initiation of the gradual plastic deformation of the specimens, deviation of the elastic energy curve from the total energy curve, reduction in growth of elastic energy, and increase in dissipated energy. After unloading the confining pressure, the growth in the elastic energy gradually slows down, whereas that of the dissipated energy increases. However, the elastic energy plummets and the dissipated energy soars with the loss of bearing capacity, leading to the instantaneous release of the energy stored in the specimens. The effect of soaking solutions on the energy of the specimens under different paths is negligible.
Table 4 shows the proportion of elastic energy and dissipated energy at the unloading points of the specimens soaked in various solutions. The table confirms the proximity of the total energy, elastic energy, and dissipated energy of the specimens at unloading points under different unloading paths, indicating the favorable homogeneity of the specimens. The ratio of elastic energy to total energy in different unloading paths decreases to different degrees from the unloading point to the failure point, in which the decreasing degree follows the following trend: loading axial pressure under unloading confining pressure (stress path II) < unloading confining pressure under constant axial pressure (stress path III) < unloading confining pressure under constant deviatoric stress (stress path IV). The proportion of dissipated energy increases by different degrees, and the increasing degree from low to high is stress path II < stress path III < stress path IV. This phenomenon can be explained by the increase axial stress during the process of stress path II, and the high releasable elastic energy stored within the specimen during compression. The ratio of elastic energy remains stable owing to the constant replenishment of the releasable elastic energy. Despite the unchanged axial stress, the stress path III process exhibits drop in the loop stress and a climb in the deviator stress, and this support the absorption and storage of elastic energy inside the specimen. In the process of stress path IV, the axial stress and loop stress dip simultaneously, and the stored elastic energy absorbed inside is less than the dissipate energy, thus the smallest proportion of the elastic energy. When the specimen reaches its ultimate bearing capacity, the elastic energy is released instantly and causes damage.
Before unloading failure, the total energy absorbed by specimens soaked in 5% NaCl under different unloading paths is always less than that of the specimens soaked in 5%K2SO4. However, with constant unloading time, the energy stored in specimens soaked in 5% K2SO4 is greater than that of specimens soaked in 5% NaCl, which explains why the 5% K2SO4 attain the energy storage limit earlier than the 5% NaCl specimens and causes failure.
5 Acoustic emission characteristics and failure modes
5.1 AE
The acoustic emission (AE) characteristics of sandstone after immersion in different solutions with different loading paths are shown in Fig. 12. The frequency of the sensors ranged from 20 to 400 kHz. The threshold, amplification of the preamplifier, and sampling rate of the acoustic emission signal (AES) testing system were set to 40 dB, 40 dB, and 1 MSPS respectively. Furthermore, to guarantee acquisition precision, PDT, HDT and HLT were set to 200 ms, 800 ms and 1000 ms respectively. The sandstone specimen under conventional triaxial compression showed low AE at the initial stage of loading, which was primarily attributed to the fact that the specimen was in the compaction stage and its internal pores were compacted. As the specimen was further compressed, it gradually yielded, at which time the AES was continuously generated because of the micro-fractures that appeared inside the specimen. The specimens under the unloading path exhibited virtually no AE generation before failure, whereas single or multiple higher AE were captured at the failure. Neither the unloading path nor the solution immersion considerably affected the AE counts of the specimens.
5.2 Failure mode
The failure modes of sandstone under different unloading confining pressure paths are shown in Fig. 13. The failure mode of the sandstone specimens in the conventional triaxial test was shear failure, and one or two shear cracks were observed on the surface of the specimens after failure. The failure mode of the sandstone specimens under the unloading confining pressure path was highly complex and different from the conventional triaxial compression test.
During the constant axials pressure and unloading confining pressure experiment, the final failure mode of the specimen was shear failure, the specimen surface had a complicated shear crack. The shear crack at both ends of the specimen had a larger angle with the horizontal direction, and the crack in the middle part had a smaller angle with the horizontal direction. In the increasing axial pressure and unloading confining pressure test, the specimens exhibited shear failure as a single linear crack with a large angle between the shear crack and the horizontal direction, and the shear crack of the sandstone after 5% K2SO4 immersion was largely parallel to the loading direction. In the constant deviatoric stress and unloading confining pressure test, the failure modes of the specimens were tenso-shear failure with one or two main cracks. Additionally, the cracks were relatively parallel to the main stress loading direction. In the unloading experiment, the circumferential stress decreased constantly during the loading procedure, the circumferential restriction of the specimen was released gradually, and the internal crack evolution of the specimen appeared highly complicated, with coexisting shear and tensile cracks.
6 Microstructure
Hydrochemical solutions exert both physical and chemical effects on sandstone the physical impact is that, the mineral components in a specimen absorb water from the solution and expand, and the water-soluble components within the specimen dissolve. The solution chemically affects the specimen by generating new minerals through the chemical reaction between ions in the soaking solution and mineral components in the specimen (Han et al. 2014). In addition, soaking affects the microstructure of sandstone by promoting changes in the mineral composition and bonding mode at the micro-level; however, at the macro level, changes in quality, porosity, and mechanical properties are observable. The mechanical properties of the specimens soaked in solutions vary owing to their diverse corrosion degrees.
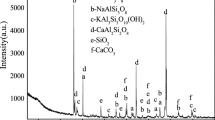
The XRD analysis was performed on the sandstone specimens before and after soaking (see Table 5). As shown in Table 5, the unsoaked sandstone specimens mainly consisted of quartz, plagioclase, calcite, and clay minerals. The compositions of those soaked in 5% NaCl remained stable, with less quartz and more clay minerals. Additionally, a small amount of halite was observed. The composition of the specimens soaked in 5% K2SO4, considerably differed from that of the dry specimen, the 5% K2SO4-soaked specimens minerals without plagioclase and calcite.
After soaking, the quartz content in all the specimens dropped to varying degrees, which is because the main mineral composition of quartz, the alkaline oxide SiO2, undergoes hydrolysis reaction in water, as shown in Eq. 7. Moreover, its solubility of SiO2 is relatively low in a neutral aqueous solution (Li et al. 2011). Plagioclase, an isomorphous series composed of two end-member components albite (NaAlSi3O8) and anorthite (CaAl2Si2O8), partially dissolves in water (Zhao 2015). According to Eqs. 8 and 9, Na+ ions dissociate from the 5% NaCl solution, which inhibits the dissolution of albite. However, the ions in the 5% K2SO4 solution combine with Ca2+ ions in anorthite forming precipitates, while the remaining components of anorthite react to generate kaolinite (Zhao 2017). In addition, calcite (CaCO3) reacts with K2SO4 during soaking to form CaSO4, which is more difficult to dissolve. The two solutions after immersion are shown in Fig. 14, notably there was a small amount of sediment in the 5% NaCl solution after immersion. Moreover, both the sediment and flocculent precipitates in the saturated 5% K2SO4 solution, which further verifies that more internal components are dissolved in sandstone specimens soaked in 5% K2SO4 solution than those soaked in 5% NaCl solution. This phenomenon directly cause the higher strain change rate of sandstone specimens soaked in 5% K2SO4 compared with sandstone specimens soaked in 5% NaCl with the decreasing of confining pressure, as well as results in a smaller unloading amount.
Figure 15 shows the SEM images of the sandstone specimens soaked in saline solutions. SiO2 mineral particles inside the specimens are apparent, and NaCl and K2SO4 crystals are observed on the surface of SiO2 mineral particles. In addition, the interconnecting material between the mineral grains of the specimens is partially eroded and dissolved, resulting in cracks. Some mineral particles in the specimen soaked in 5% K2SO4 further react with ions in the solution to produce cracks.
7 Conclusions
This study analyzed the mechanics, deformation, failure and energy evolution characteristics of sandstone specimens under different soaking conditions during the process of unloading confining pressure, and investigated the effects of different solutions on the confining pressure unloading characteristics of sandstone specimens combined with the microscopic composition and structure. The main findings of this study are as follows:
-
(1)
The difference between the stress–strain curves of sandstone immersed in different solutions under different stress paths was primarily reflected in the unloading period. The unloading quantity of sandstone specimens immersed in 5% NaCl was higher than that of specimens immersed in 5% K2SO4, and the unloading capacity generally followed the sequence of stress paths II < stress paths III < stress paths IV.
-
(2)
The deformation modulus and Poisson's ratio of the specimens under different unloading paths exhibited exponential changes with the unloading ratio. The deformation modulus of the 5% NaCl-soaked sandstone under different unloading paths was higher than that of the 5% K2SO4-soaked specimens; however, the change in Poisson's ratio followed the opposing trend.
-
(3)
Through energy dissipation analysis, the total energy absorbed by the 5% NaCl-immersed specimens under different unloading paths was often lower than that of the 5% K2SO4-immersed sandstone. The AE of the sandstone specimens under the unloading path had only one or more loud emissions at the failure site.
-
(4)
Compared with that of dry sandstone, the mineral composition of the sandstone immersed in the brine solution decreased in quartz content and increased in clay minerals. After immersion, the cement between the mineral particles of the specimens was partially eroded and dissolved, resulting in internal micro-cracks. Some mineral particles of the sandstone specimens immersed in 5% K2SO4 reacted with the ions in the solution causing the appearance of cracks.
References
Chen ZQ, Li TB, Chen GQ, Ma CC (2016) Experimental study on variation of sandstone energy consumption under different stress paths. Eng Mech 33(6):120–128
Gao CY, Xu J, He P, Liu JF (2005) Study on mechanical properties of magmatic rock during loading and unloading. Chin J Rock Mech Eng 24(3):456–460
Haider MG, Jay GS, Ranjith PG (2013) Strength of geopolymer cured in saline water in ambient conditions. Fuel 107(may):34–39
Han TL, Chen YS, Shi JP, Yu Z, He MM (2013) Experimental study on the effect of hydrochemical corrosion on mechanical properties of sandstone. Chin J Rock Mech Eng 32(S2):3064–3072
Han TL, Shi JP, Chen YS, Ma WT (2014) Experimental study on influence of Chemical Corrosion on physical and mechanical properties of sandstone. J **’an Univ Technol 30(1):34–39
Hu YY (2016) Study on unloading mechanical properties of bedding sandstone under water-rock interaction in the Three Gorges Reservoir Area. China Three Gorges University, Yichang
Li T (2015) Mechanical properties and unloading constitutive model of siltstone under different stress paths. China University of Mining and Technology, Xuzhou
Li JL, Wang RH, Jiang YZ, Liu J, Chen X (2010) Experimental study on mechanical properties of sandstone under triaxial Unloading. Chin J Rock Mech Eng 29(10):2034–2041
Li P, Liu J, Li GH, Zhu JB, Liu SG (2011) Effect of hydrochemistry on shear strength of sandstone. Rock Soil Mech 32(2):380–386
Li HG, Shao X, Yao HY, Wu P (2016b) Experimental study on mechanical properties of soft sandstone under loading and unloading by water. J Hefei Univ Technol (Nat Sci) 39(3):360–363
Li DY, Sun Z, Li XB, **e T (2016a) Mechanical response and failure characteristics of granite under triaxial loading and unloading under different stress paths. Chin J Rock Mech Eng 35(S2):3449–3457
Li DY, Sun Z, **e T, Li XB, Ranjith PG (2017) Energy evolution characteristics of hard rock during triaxial failure with different loading and unloading paths. Eng Geol 228:270–281
Lin YT, He JQ, Ye MC (2002a) Study on the distribution type and quality of potassium resources in brine in Sichuan Basin. Chem Miner Geol 04:215–247
Lin YT, He JQ, Ye MC (2002b) Study on distribution types and quality of potassium resources in brine in Sichuan basin. Geol Chem Miner 24(4):215–221
Nasvi MCM, Ranjith PG, Sanjayan J et al (2014) Mechanical behaviour of wellbore materials saturated in brine water with different salinity levels. Energy 66(1):239–249
Rathnaweera TD, Ranjith PG, Perera MSA (2014) Salinity-dependent strength and stress–strain characteristics of reservoir rocks in deep saline aquifers: an experimental study. Fuel 122:1–11
Rathnaweera TD, Ranjith PG, Perera MSA et al (2015) Non-linear stress–strain behaviour of reservoir rock under brine saturation: an experimental study. Measurement 71:56–72
Wang W, Li XH, Zhu QZ, Shi C, Xu WY (2017) Experimental study on the effect of hydrochemical corrosion on mechanical properties of sand slates. Rock Soil Mech 38(9):2559–2573
**e HP, Ju Y, Li LY (2005) Rock strength and global failure criterion based on energy dissipation and release principle. Chin J Rock Mech Eng 24(17):3003–3010
Yang SQ, Huang YH, Ranjith PG (2018) Failure mechanical and acoustic behavior of brine saturated-sandstone containing two pre-existing flaws under different confining pressures. Eng Fract Mech 193:108–121
Yang SQ, Xu SB, Liu Z (2021) Deep sandstone mechanics and seepage characteristics of saline environment test research. J Rock Mech Eng 7:1–13. https://doi.org/10.13722/j.cnki.jrme.2021.0326
Zhao SR (2015) Concise mineralogy, vol 6. China University of Geosciences Press, Wuhan, pp 43–44
Zhao SR (2017) Crystallography and mineralogy, vol 5, 3s edn. Higher Education Press, Bei**g, p 366
Zhao GY, Dai B, Dong LJ, Yang C (2015) Energy conversion of rocks in process of unloading confining pressure under different unloading paths. Trans Nonferrous Met Soc China 25(5):1626–1632
Zhu ZH, Yu LY, Li JL, Meng QB, Sui B, Zhang ZQ (2020) Deformation evolution and failure energy dissipation characteristics of marble unloaded before peak. J China Coal Soc 45(S1):181–190
Scrivano S, Gaggero L (2020) An experimental investigation into the salt-weathering susceptibility of building limestones. Rock Mech Rock Eng (prepublish)
Wang YM, Han LJ, Han GL, Zhang HQ, Qu T (2007) Test of surrounding rock loose zone and bolt-grouting support practice in complex Strata. In: Proceedings of the first Chinese academic conference on rock mechanics and engineering, pp 272–278
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant No. 42077231] and Fundamental Research Funds for Central Universities [Grant No. 2020ZDPYMS34].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, SQ., Xu, SB., Liu, Z. et al. Experimental investigation on the triaxial unloading mechanical characteristics of sandstone immersed in different brines. Geomech. Geophys. Geo-energ. Geo-resour. 9, 71 (2023). https://doi.org/10.1007/s40948-023-00602-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40948-023-00602-7