Abstract
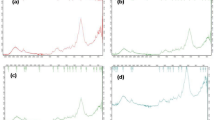
Recent decades have witnessed an enormous increase in concentrations of dye effluent and its harmful effect on aquatic environment. The treatment of dye effluent before discharging in aquatic ecosystem is extremely important from the environmental viewpoint because of its toxic, carcinogenic, and mutagenic nature. The objective of the current study was to evaluate the feasibility of dead biomass of aquatic weeds namely, Brachiaria mutica and Cyperus rotundus as adsorbents for the removal of methylene blue dye from aqueous solution. The batch study was performed to study the effect of various parameters such as pH (1–8), adsorbent dose (0.2–1.6 g/100 ml), initial dye concentration (10–80 ppm), contact time (15–120 min), agitation speed (50–250 rpm), and temperature (10–60 °C). The Fourier Transform Infrared Spectroscopy (FTIR) clearly indicated slightly shifting in peak after treatment with methylene blue. Removal efficiencies of methylene blue were 90% for B. mutica and 94% for C. rotundus, achieved at pH of 6 (for B. mutica) and 8 (for C. rotundus), adsorbent dose of 0.8 g (for B. mutica) and 1 g (for C. rotundus), concentration of 50 ppm, contact time of 60 min, shaking rate of 150 rpm, and temperature of 40℃. Maximum adsorption capacities were 21.49 mg/g for B. mutica and 21.94 mg/g for C. rotundus. Freundlich isotherm (R2 = 0.818 for B. mutica and 0.945 for C. rotundus) fitted well to experimental data in comparison to Langmuir isotherm. Whereas in case of adsorption kinetics, pseudo-second order was better fitted as compared to first order for both the adsorbents. Thermodynamics parameters show that the adsorption process is endothermic and non-spontaneous at low temperature for both the adsorbents. Thus, present study suggested that both the adsorbents can be effectively utilized for treatment of dye effluent.













Similar content being viewed by others
References
Akkaya G, Güzel F (2014) Application of some domestic wastes as new low-cost biosorbents for removal of methylene blue: kinetic and equilibrium studies. Chem Eng Commun 201(4):557–578. https://doi.org/10.1080/00986445.2013.780166
Ashraf MY, Awan AR, Mahmood K (2012) Rehabilitation of saline ecosystems through cultivation of salt tolerant plants. Pak J Bot 44:69–75
Asif TM, Bhatt HN, Iqbal M (2016) Solar red and brittle blue direct dyes adsorption onto Eucalyptus angopHoroides bark: equilibrium, kinetics and thermodynamic studies. J Environ Chem Engg 4(2):2431–2439. https://doi.org/10.1016/j.jece.2016.04.020
Balarak D, Bazrafshan E, Mahdavi Y (2015) A study on Kinetics modeling and isotherms for removal of Cr (VI) from aqueous solution by modified Cyperus rotundus Weed biomass. J Environ Health Res 1(3):207–216
Bayomie OS, Kandeel H, Shoeib T, Yang H, Youssef N, El-Sayed MM (2020) Novel approach for effective removal of methylene blue from water using fava bean peels waste. Sci Rep 10(1):1–10
Bhatti HN, Safa Y, Yakout SM, Shair OH, Iqbal M, Nazir A (2020) Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: adsorption/desorption, kinetics and recycling studies. Int J Biol Macromol 150:861–870. https://doi.org/10.1016/j.ijbiomac.2020.02.093
Chakraborty TK, Islam MS, Zaman S, Kabir AHME, Ghosh GC (2020) Jute (Corchorus olitorius) stick charcoal as a low-cost adsorbent for the removal of methylene blue from aqueous solution. SN Appl Sci 2(4):1–10. https://doi.org/10.1007/s42452-020-2565-y
Freundlich H (1906) Over the adsorption in solution. J PHys Chem 57:358–471
Ghafar HHA, Salem T, Radwan EK, El-Sayed AA, Embaby MA, Salama M (2017) Modification of waste wool fiber as low cost adsorbent for the removal of methylene blue from aqueous solution. Egypt J Chem 60(3):395–406
Gong R, Li M, Yang C, Sun Y, Chen J (2005) Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J Hazard Mater 121(1–3):247–250. https://doi.org/10.1016/j.jhazmat.2005.01.029
Güzel F, Sayğılı H, Sayğılı GA, Koyuncu F (2015) New low-cost nanoporous carbonaceous adsorbent developed from carob (Ceratonia siliqua) processing industry waste for the adsorption of anionic textile dye: characterization, equilibrium and kinetic modeling. J Mol Liq 206:244–255. https://doi.org/10.1016/j.molliq.2015.02.037
Islam MR, Mostafa MG (2022) Adsorption kinetics, isotherms and thermodynamic studies of methyl blue in textile dye effluent on natural clay adsorbent. Sustain Water Resour Manag 8:52. https://doi.org/10.1007/s40899-022-00640-1
Kallel F, Chaari F, Bouaziz F, Bettaieb F, Ghorbel R, Chaabouni SE (2016) Sorption and desorption characteristics for the removal of a toxic dye, methylene blue from aqueous solution by a low cost agricultural by-product. J Mol Liq 219:279–288. https://doi.org/10.1016/j.molliq.2016.03.024
Kerkez Đ, Bečelić-Tomin M, Milidrag GP, Gvoić V, Mandić, AK, Maćerak AL Pilipović, DT (2020) Treatment of wastewater containing printing dyes: summary and perspectives. https://doi.org/10.24867/GRID-2020-p31
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Lalnunhlimi S, Krishnaswamy V (2016) Decolorization of azo dyes (Direct Blue 151 and Direct Red 31) by moderately alkalipHilic bacterial consortium. Braz J Microbiol 47:39–46. https://doi.org/10.1016/j.bjm.2015.11.013
Langmuir (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38(11):2221–2295
Lim LB, Priyantha N, Chieng HI, Dahri MK (2016) Artocarpus camansi Blanco (Breadnut) core as low-cost adsorbent for the removal of methylene blue: equilibrium, thermodynamics, and kinetics studies. Desalin Water Treat 57(12):5673–5685. https://doi.org/10.1080/19443994.2015.1007088
Mousa KM, Taha AH (2016) Adsorption of reactive blue dye onto natural and modifed wheat straw. Am J Chem Eng 4(1):9–15. https://doi.org/10.11648/j.ajche.20160401.12
Nayeri D, Mousavi SA (2020) Dye removal from water and wastewater by nanosized metal oxides-modified activated carbon: a review on recent researches. J Environ Health Sci 18(2):1671–1689
Ngah WW, Hanafiah MAKM (2008) Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: Kinetic, equilibrium and thermodynamic studies. Biochem Eng 9(3):521–530. https://doi.org/10.1016/j.bej.2007.11.006
Orozco RS, Martínez-Juan M, García-Sánchez JJ, Ureña-Núñez F (2018) Removal of methylene blue from aqueous solution using Typha stems and leaves. BioResources 13(1):1696–1710
Papegowda PK, Syed AA (2017) Isotherm, kinetic and thermodynamic studies on the removal of methylene blue from aqueous solution using saw palmetto spent. Int J Environ Res 11(1):91–98
Parvin S, Rahman MW, Saha I, Alam MJ, Khan MMR (2019) Coconut tree bark as a potential low-cost adsorbent for the removal of methylene blue from wastewater. Desalin Water Treat 146:385–392
Pathania D, Sharma S, Singh P (2017) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab J Chem 10:S1445–S1451. https://doi.org/10.1016/j.arabjc.2013.04.021
Patil S, Renukdas S, Patel N (2011) Removal of methylene blue, a basic dye from aqueous solutions by adsorption using teak tree (Tectona grandis) bark powder. Int J Environ Sci 1(5):711
Rahimian R, Zarinabadi S (2020) A review of studies on the removal of methylene blue from industrial wastewater using activated carbon adsorbents made from almond bark. Prog Chem Biochem Res 3(3):251–268
Roozkhosh M, Eslami SV, Jami Al-ahmadi M (2015) Effect of soil solarization on the control of different purple nutsedge (Cyperus rotundus L.). Ecotypes. 28(4):579–588
Salem A, Fayed IA, Nahass TNME, Dawood M (2018) A comparative study for adsorption of methylene blue from wastewater on to three different types of rice ash. J Pharm Appl Chem 4(1):30–39
Sarath Babu B, Yamini G (2020) Removal of Methylene blue by using lemon leaf powder as an adsorbent. In: Ghosh S, Saha P, Francesco Di M (eds) Recent trends in waste water treatment and water resource management. Springer, Singapore. https://doi.org/10.1007/978-981-15-0706-9_10
Shakoor S, Nasar A (2016) Removal of methylene blue from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J Taiwan Inst Chem Eng 66:154–163
Shakoor S, Nasar A (2017) Adsorptive treatment of hazardous methylene blue from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw Sustain Dev 5:152–159. https://doi.org/10.1016/j.gsd.2017.06.005
Siddiqui SI, Rathi G, Chaudhry SA (2018) Acid washed black cumin seed powder preparation for adsorption of methylene blue from aqueous solution: thermodynamic, kinetic and isotherm studies. J Mol Liq 264:275–284. https://doi.org/10.1016/j.molliq.2018.05.065
Soni M, Sharma AK, Srivastava JK, Yadav JS (2012a) Adsorptive removal of methylene blue from an aqueous solution using water hyacinth root powder as a low cost adsorbent. Int J Chem Sci 3(3):338–345
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta PHysiochim URSS 12:217–225
Wei F, Shahid MJ, Alnusairi GS, Afzal M, Khan A, El-Esawi MA, Ali S (2020) Implementation of floating treatment wetlands for textile wastewater management: a review. Sustainability 12(14):580. https://doi.org/10.3390/su12145801
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Yaseen DA, Scholz M (2019) Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol 16(2):1193–1226. https://doi.org/10.1007/s13762-018-2130-z
Acknowledgements
The authors are thankful to the Department of Botany, Maharshi Dayanand University for the identification of aquatic plant species, and the Department of Genetics for providing the FTIR facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arora, D., Arora, A., Singh, A. et al. Usability of Brachiaria mutica (para grass) and Cyperus rotundus (nut grass) as bioadsorbents for the removal of methylene blue from aqueous solution: isotherms, kinetics, and thermodynamics studies. Sustain. Water Resour. Manag. 8, 139 (2022). https://doi.org/10.1007/s40899-022-00734-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-022-00734-w