Abstract
Purpose
Alzheimer’s disease (AD) is the most common type of dementia, and its early diagnosis has become a crucial issue. Machine learning provides a systematic and objective approach in classification. Currently, there are many studies using several kinds of neuroimaging modalities to perform classification in dementia. Support vector machine (SVM) is one of machine learning based classification algorithm which is able to retain favorable classification accuracy even with small sample sizes. Our aim is to investigate the feasibility of using dual PET biomarkers in combination with SVM for AD diagnosis in small sample sizes.
Methods
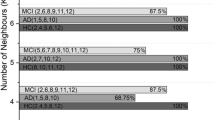
This study collected PET (18F-FDG and 11C-PiB) and T1 MRI image of 79 subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database, including 20 AD, 27 mild cognitive impairment (MCI) subjects, and 32 normal controls (NCs), and performed classification using the SVM algorithm with the quantification of the two PET biomarkers, and finally compared the classification results of each brain region.
Results
In the classification between diseased (AD and MCI) and NC group, we found that the accuracy, sensitivity and specificity mean in temporal cortex are the highest.
Conclusions
Overall, using dual PET biomarkers in combination with SVM shows a certain feasibility and clinical value in the diagnosis of AD, especially in the temporal cortex.



Similar content being viewed by others
References
Jack, C. R. Jr., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9(1), 119–128.
Brookmeyer, R., et al. (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & Dementia, 3(3), 186–191.
McKhann, G. M., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269.
Nelissen, N., et al. (2009). Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. Journal of Nuclear Medicine, 50(8), 1251–1259.
Klunk, W. E., et al. (2005). Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer’s disease brain but not in transgenic mouse brain. Journal of Neuroscience, 25(46), 10598–10606.
Klunk, W. E., et al. (2004). Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 55(3), 306–319.
Buckner, R. L., et al. (2005). Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience, 25(34), 7709–7717.
Jagust, W. J., et al. (1985). Positron emission tomography with [18F] fluorodeoxyglucose differentiates normal pressure hydrocephalus from Alzheimer-type dementia. Journal of Neurology, Neurosurgery & Psychiatry, 48(11), 1091–1096.
Hoffman, J. M., et al. (2000). FDG PET imaging in patients with pathologically verified dementia. Journal of Nuclear Medicine, 41(11), 1920–1928.
Ishii, K., et al. (2001). Statistical brain map** of 18F-FDG PET in Alzheimer’s disease: Validation of anatomic standardization for atrophied brains. Journal of Nuclear Medicine, 42(4), 548–557.
Matsunari, I., et al. (2007). Comparison of 18F-FDG PET and optimized voxel-based morphometry for detection of Alzheimer’s disease: Aging effect on diagnostic performance. Journal of Nuclear Medicine, 48(12), 1961–1970.
Minoshima, S., et al. (1995). A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. Journal of Nuclear Medicine, 36(7), 1238–1248.
Villemagne, V. L., et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: AA prospective cohort study. The Lancet Neurology, 12(4), 357–367.
Mosconi, L., et al. (2008). Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. Journal of Nuclear Medicine, 49(3), 390–398.
Petersen, R. C., et al. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308.
Petersen, R. C., et al. (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985–1992.
Tierney, M., et al. (1996). Prediction of probable Alzheimer’s disease in memory-impaired patients: A prospective longitudinal study. Neurology, 46(3), 661–665.
Wu, L., et al. (2012). Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS ONE, 7(10), e47905.
Edison, P., et al. (2007). Amyloid, hypometabolism, and cognition in Alzheimer disease: An [11C] PIB and [18F] FDG PET study. Neurology, 68(7), 501–508.
Meyer, P. T., et al. (2011). Dual-biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11C-labeled Pittsburgh compound B. Journal of Nuclear Medicine, 52(3), 393–400.
Klöppel, S., et al. (2008). Automatic classification of MR scans in Alzheimer’s disease. Brain, 131(3), 681–689.
Hinrichs, C., et al. (2009). Spatially augmented LPboosting for AD classification with evaluations on the ADNI dataset. Neuroimage, 48(1), 138–149.
Termenon, M., et al. (2012). A two stage sequential ensemble applied to the classification of Alzheimer’s disease based on mri features. Neural Processing Letters, 35(1), 1–12.
Adaszewski, S., et al. (2013). How early can we predict Alzheimer’s disease using computational anatomy? Neurobiology of Aging, 34(12), 2815–2826.
Plant, C., et al. (2010). Automated detection of brain atrophy patterns based on MRI for the prediction of Alzheimer’s disease. Neuroimage, 50(1), 162–174.
Salvatore, C., et al. (2015). Magnetic resonance imaging biomarkers for the early diagnosis of Alzheimer’s disease: A machine learning approach. Frontiers in Neuroscience, 9, 307.
Misra, C., et al. (2009). Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: Results from ADNI. Neuroimage, 44(4), 1415–1422.
Eskildsen, S. F., et al. (2013). Prediction of Alzheimer’s disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. Neuroimage, 65, 511–521.
Cabral, C., et al. (2015). Predicting conversion from MCI to AD with FDG-PET brain images at different prodromal stages. Computers in Biology and Medicine, 58, 101–109.
Vandenberghe, R., et al. (2013). Binary classification of 18F-flutemetamol PET using machine learning: Comparison with visual reads and structural MRI. Neuroimage, 64, 517–525.
Shao, Y., et al. (2012). Comparison of support vector machine, neural network, and CART algorithms for the land-cover classification using limited training data points. ISPRS Journal of Photogrammetry and Remote Sensing, 70, 78–87.
Gispert, J., et al. (2003). Influence of the normalization template on the outcome of statistical parametric map** of PET scans. Neuroimage, 19(3), 601–612.
Fein, G., et al. (2006). Statistical parametric map** of brain morphology: Sensitivity is dramatically increased by using brain-extracted images as inputs. Neuroimage, 30(4), 1187–1195.
Ashburner, J., et al. (1999). Nonlinear spatial normalization using basis functions. Human Brain Map**, 7(4), 254–266.
Alemán-Gómez, Y. (2006). IBASPM: toolbox for automatic parcellation of brain structures. In 12th Annual Meeting of the Organization for Human Brain Map**. June 11–15, 2006. Florence, Italy.
Tzourio-Mazoyer, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289.
Rolls, E. T., et al. (2015). Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage, 122, 1–5.
Ng, S., et al. (2007). Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. Journal of Nuclear Medicine, 48(4), 547–552.
Lin, K.-J., et al. (2016). Imaging characteristic of dual-phase 18 F-florbetapir (AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer’s disease and mild cognitive impairment. European Journal of Nuclear Medicine and Molecular Imaging, 43(7), 1304–1314.
Brown, R. K., et al. (2014). Brain PET in suspected dementia: patterns of altered FDG metabolism. Radiographics, 34(3), 684–701.
Minoshima, S., et al. (1995). Preserved pontine glucose metabolism in Alzheimer disease: A reference region for functional brain image (PET) analysis. Journal of Computer Assisted Tomography, 19(4), 541–547.
Mosconi, L., et al. (2007). Quantitation, regional vulnerability, and kinetic modeling of brain glucose metabolism in mild Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging, 34(9), 1467–1479.
Ishii, K., et al. (2015). Regional glucose metabolic reduction in dementia with Lewy bodies is independent of amyloid deposition. Annals of Nuclear Medicine, 29(1), 78–83.
Melgani, F., et al. (2004). Classification of hyperspectral remote sensing images with support vector machines. IEEE Transactions on Geoscience and Remote Sensing, 42(8), 1778–1790.
Zhang, Y., et al. (2015). Detection of Alzheimer’s disease by displacement field and machine learning. PeerJ, 3, e1251.
Johnson, K. A., et al. (2007). Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 62(3), 229–234.
Rabinovici, G., et al. (2007). 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology, 68(15), 1205–1212.
Suotunen, T., et al. (2010). Visual assessment of [11 C] PIB PET in patients with cognitive impairment. European Journal of Nuclear Medicine and Molecular Imaging, 37(6), 1141–1147.
Tolboom, N., et al. (2010). Molecular imaging in the diagnosis of Alzheimer’s disease: Visual assessment of [11C] PIB and [18F] FDDNP PET images. Journal of Neurology, Neurosurgery & Psychiatry, 81(8), 882–884.
Nordberg, A., et al. (2013). A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging, 40(1), 104–114.
Rowe, C. C., et al. (2010). Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging, 31(8), 1275–1283.
Ewers, M., et al. (2012). CSF biomarker and PIB-PET–derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects. Cerebral Cortex, 22(9), 1993–2004.
Acknowledgements
The authors are grateful to the grant support from Ministry of Science and Technology, Taiwan, R.O.C. under Grant No. MOST 108-2314-B-075-007. Data used in preparation of this article were obtained from the Alzheimer’s disease Neuroimaging Initiative (ADNI) database (https://www.adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yang, BH., Chen, JC., Chou, WH. et al. Classification of Alzheimer’s Disease from 18F-FDG and 11C-PiB PET Imaging Biomarkers Using Support Vector Machine. J. Med. Biol. Eng. 40, 545–554 (2020). https://doi.org/10.1007/s40846-020-00548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-020-00548-1