Abstract
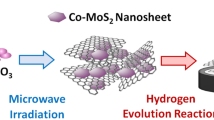
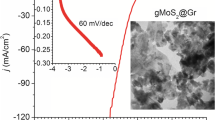
Low-cost, highly efficient catalysts for hydrogen evolution reaction (HER) are very important to advance energy economy based on clean hydrogen gas. Intensive studies on two-dimensional molybdenum disulfides (2D MoS2) have been conducted due to their remarkable catalytic properties. However, most of the reported syntheses are time consuming, complicated and less efficient. The present work demonstrates the production of MoS2/graphene catalyst via an ultra-fast (60 s) microwave-initiated approach. High specific surface area and conductivity of graphene delivers a favorable conductive network for the growth of MoS2 nanosheets, along with rapid charge transfer kinetics. As-produced MoS2/graphene nanocomposites exhibit superior electrocatalytic activity for the HER in acidic medium, with a low onset potential of 62 mV, high cathodic currents and a Tafel slope of 43.3 mV/decade. Beyond excellent catalytic activity, MoS2/graphene reveals long cycling stability with a very high cathodic current density of around 1000 mA cm−2 at an overpotential of 250 mV. Moreover, the MoS2/graphene-catalyst exhibits outstanding HER activities in a temperature range of 30 to 120°C with low activation energy of 36.51 kJ mol−1, providing the opportunity of practical scalable processing.
摘要
用于析氢反应(HER)的低成本、高效能催化剂对于推进基 于清洁氢气的能源工业非常重要. 二维二硫化钼(MoS2)具有显著的 催化性能, 因而已被人们广泛深入研究. 然而, 大多数现有的合成方 法耗时、复杂且效率较低. 本文通过超快(60秒)微波引发的方法生 产MoS2/石墨烯催化剂. 石墨烯的高比表面积和导电性为MoS2纳米 片的生长提供了有利的导电网络和快速电荷转移动力. 文中制备 的MoS2/石墨烯纳米复合材料在酸性介质中对HER表现出优异的 电催化活性, 具有62 mV的低起始电位, 高阴极电流和43.3mV/dec 的Tafel斜率. 除了优异的催化活性外, MoS2/石墨烯还具有较长的 循环稳定性, 在2 5 0 mV的过电位下阴极电流密度高达 1000 mA cm−2. 此外, MoS2/石墨烯催化剂在30–120°C范围内具有 出色的HER活性和36.51 kJ mol−1的低活化能, 提供了潜在的大批量 生产和制备的机会.
Similar content being viewed by others
References
Mazloomi K, Gomes C. Hydrogen as an energy carrier: Prospects and challenges. Renew Sustain Energy Rev, 2012, 16: 3024–3033
Turner JA. Sustainable hydrogen production. Science, 2004, 305: 972–974
Ball M, Wietschel M. The future of hydrogen—opportunities and challenges. Int J Hydrogen Energy, 2009, 34: 615–627
Morales-Guio CG, Stern LA, Hu X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem Soc Rev, 2014, 43: 6555
Thoi VS, Sun Y, Long JR, et al. Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem Soc Rev, 2013, 42: 2388–2400
Zou X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev, 2015, 44: 5148–5180
Marinović V, Stevanović J, Jugović B, et al. Hydrogen evolution on Ni/WC composite coatings. J Appl Electrochem, 2006, 36: 1005–1009
Krstajic N, Jovic V, Gajickrstajic L, et al. Electrodeposition of Ni-Mo alloy coatings and their characterization as cathodes for hydrogen evolution in sodium hydroxide solution. Int J Hydrogen Energy, 2008, 33: 3676–3687
Han Q. Hydrogen evolution reaction on amorphous Ni-S-Co alloy in alkaline medium. Int J Hydrogen Energy, 2003, 28: 1345–1352
Evans DJ, Pickett CJ. Chemistry and the hydrogenases. Chem Soc Rev, 2003, 32: 268
Rees DC, Howard JB. The interface between the biological and inorganic worlds: Iron-sulfur metalloclusters. Science, 2003, 300: 929–931
Morales-Guio CG, Hu X. Amorphous molybdenum sulfides as hydrogen evolution catalysts. Acc Chem Res, 2014, 47: 2671–2681
Yan Y, **a BY, Xu Z, et al. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal, 2014, 4: 1693–1705
Laursen AB, Kegnæs S, Dahl S, et al. Molybdenum sulfides—efficient and viable materials for electro- and photoelectrocatalytic hydrogen evolution. Energy Environ Sci, 2012, 5: 5577
Benck JD, Chen Z, Kuritzky LY, et al. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: Insights into the origin of their catalytic activity. ACS Catal, 2012, 2: 1916–1923
Jaramillo TF, Jørgensen KP, Bonde J, et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science, 2007, 317: 100–102
Kong D, Wang H, Cha JJ, et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett, 2013, 13: 1341–1347
Li Y, Wang H, **e L, et al. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J Am Chem Soc, 2011, 133: 7296–7299
Yang Y, Fei H, Ruan G, et al. Edge-oriented MoS2 nanoporous films as flexible electrodes for hydrogen evolution reactions and supercapacitor devices. Adv Mater, 2014, 26: 8163–8168
Voiry D, Salehi M, Silva R, et al. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett, 2013, 13: 6222–6227
Deng J, Li H, **. Energy Environ Sci, 2015, 8: 1594–1601
Wang H, Lu Z, Xu S, et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc Natl Acad Sci USA, 2013, 110: 19701–19706
Gao MR, Liang JX, Zheng YR, et al. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat Commun, 2015, 6: 5982
Zhu Y, Murali S, Cai W, et al. Graphene and graphene oxide: Synthesis, properties, and applications. Adv Mater, 2010, 22: 3906–3924
**ang Q, Yu J, Jaroniec M. Graphene-based semiconductor photocatalysts. Chem Soc Rev, 2012, 41: 782–796
Firmiano EGS, Cordeiro MAL, Rabelo AC, et al. Graphene oxide as a highly selective substrate to synthesize a layered MoS2 hybrid electrocatalyst. Chem Commun, 2012, 48: 7687
Tang YJ, Wang Y, Wang XL, et al. Molybdenum disulfide/nitrogen-doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution. Adv Energy Mater, 2016, 6: 1600116
Sun Y, Hu X, Luo W, et al. Self-assembled hierarchical MoO2/graphene nanoarchitectures and their application as a high-performance anode material for lithium-ion batteries. ACS Nano, 2011, 5: 7100–7107
Bhaskar A, Deepa M, Rao TN, et al. Enhanced nanoscale conduction capability of a MoO2/graphene composite for high performance anodes in lithium ion batteries. J Power Sources, 2012, 216: 169–178
Zhao Y, **e X, Zhang J, et al. MoS2 nanosheets supported on 3D graphene aerogel as a highly efficient catalyst for hydrogen evolution. Chem Eur J, 2015, 21: 15908–15913
Murugesan S, Kearns P, Stevenson KJ. Electrochemical deposition of germanium sulfide from room-temperature ionic liquids and subsequent Ag do** in an aqueous solution. Langmuir, 2012, 28: 5513–5517
Jiang Y, Zhu YJ. Microwave-assisted synthesis of sulfide M2S3 (M = Bi, Sb) nanorods using an ionic liquid. J Phys Chem B, 2005, 109: 4361–4364
Zhang X, Liu Z. Recent advances in microwave initiated synthesis of nanocarbon materials. Nanoscale, 2012, 4: 707–714
Liu Z, Zhang L, Poyraz S, et al. An ultrafast microwave approach towards multi-component and multi-dimensional nanomaterials. RSC Adv, 2014, 4: 9308
Anantharaj S, Kennedy J, Kundu S. Microwave-initiated facile formation of Ni3Se4 nanoassemblies for enhanced and stable water splitting in neutral and alkaline media. ACS Appl Mater Interfaces, 2017, 9: 8714–8728
Dong GH, Zhu YJ, Chen LD. Microwave-assisted rapid synthesis of Sb2Te3 nanosheets and thermoelectric properties of bulk samples prepared by spark plasma sintering. J Mater Chem, 2010, 20: 1976
Zhang Y, Qiao ZP, Chen XM. Microwave-assisted elemental direct reaction route to nanocrystalline copper chalcogenides CuSe and Cu2Te. J Mater Chem, 2002, 12: 2747–2748
Liu Z, Zhang L, Wang R, et al. Ultrafast microwave nano-manufacturing of fullerene-like metal chalcogenides. Sci Rep, 2016, 6: 22503
Li H, Zhang Q, Yap CCR, et al. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv Funct Mater, 2012, 22: 1385–1390
Zhang X, Meng F, Mao S, et al. Amorphous MoSxCly electro-catalyst supported by vertical graphene for efficient electrochemical and photoelectrochemical hydrogen generation. Energy Eniron Sci, 2015, 8: 862–868
Lee C, Yan H, Brus LE, et al. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano, 2010, 4: 2695–2700
Mandal M, Ghosh D, Kalra SS, Das CK. High performance supercapacitor electrode material based on flower like MoS2/reduced graphene oxide nanocomposite. Int J Latest Res Technol, 2014, 3: 65–69
Khan M, Yousaf AB, Chen M, et al. Molybdenum sulfide/graphene-carbon nanotube nanocomposite material for electro-catalytic applications in hydrogen evolution reactions. Nano Res, 2016, 9: 837–848
Liu Y, Zhao Y, Jiao L, et al. A graphene-like MoS2/graphene nanocomposite as a highperformance anode for lithium ion batteries. J Mater Chem A, 2014, 2: 13109–13115
Sheng W, Gasteiger HA, Shao-Horn Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. J Electrochem Soc, 2010, 157: B1529
Thomas JGN. Kinetics of electrolytic hydrogen evolution and the adsorption of hydrogen by metals. Trans Faraday Soc, 1961, 57: 1603–1611
Huang Z, Luo W, Ma L, et al. Dimeric [Mo2S12]2- cluster: A molecular analogue of MoS2 edges for superior hydrogen-evolution electrocatalysis. Angew Chem Int Ed, 2015, 54: 15181–15185
Kong D, Wang H, Lu Z, et al. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J Am Chem Soc, 2014, 136: 4897–4900
Tang YJ, Gao MR, Liu CH, et al. Porous molybdenum-based hybrid catalysts for highly efficient hydrogen evolution. Angew Chem Int Ed, 2015, 54: 12928–12932
McCrory CCL, Jung S, Ferrer IM, et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc, 2015, 137: 4347–4357
Liao L, Zhu J, Bian X, et al. MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv Funct Mater, 2013, 23: 5326–5333
Zheng X, Xu J, Yan K, et al. Space-confined growth of MoS2 nanosheets within graphite: The layered hybrid of MoS2 and graphene as an active catalyst for hydrogen evolution reaction. Chem Mater, 2014, 26: 2344–2353
Li G, Zhang D, Yu Y, et al. Activating MoS2 for pH-universal hydrogen evolution catalysis. J Am Chem Soc, 2017, 139: 16194–16200
Liu Y, Wu J, Hackenberg KP, et al. Self-optimizing, highly surface-active layered metal dichalcogenide catalysts for hydrogen evolution. Nat Energy, 2017, 2: 17127
Santos DMF, Sequeira CAC, Macciò D, et al. Platinum-rare earth electrodes for hydrogen evolution in alkaline water electrolysis. Int J Hydrogen Energy, 2013, 38: 3137–3145
Durst J, Simon C, Hasché F, et al. Hydrogen oxidation and evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd electrocatalysts in acidic media. J Electrochem Soc, 2014, 162: F190–F203
Acknowledgements
This work was supported by Auburn University-Intramural Grants Program (AU-IGP). Authors would like to thank Ali Rashti and Tae-Sik Oh for their help with BET analysis. Authors also thank Horyun Lee for the help with Raman analysis.
Author information
Authors and Affiliations
Contributions
Sarwar S performed the electrochemical characterizations, analyzed the data and wrote this paper with the input from all authors. Nautiyal A and Cook J helped with useful suggestions and guidance. Li J and Wang R contributed with SEM and EDS tests. Uprety S and Park M helped with the collection of Raman data and Bozack MJ contributed with XPS tests. Yuan Y and Yassar R were involved with TEM tests and revised the manuscript. Zhang X supervised the entire project and revised the manuscript.
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Shatila Sarwar received her BSc degree in chemical engineering at Bangladesh University of Engineering & Technology (BUET). She is now a PhD candidate in Prof. **nyu Zhang’s Laboratory at the Department of Chemical Engineering, Auburn University, USA. Her current research focuses on the microwave-initiated synthesis of transition metal dichalcogenides for electrochemical energy conversion and storage applications.
**nyu Zhang studied in Chemistry Department at the University of Texas at Dallas (UTD) under the supervision of Professors Alan G. MacDiar-mid and Sanjeev K. Manohar. After receiving his PhD degree in 2005, he started his postdoctoral stay at the University of Massachusetts Lowell. He started his career at Auburn University in 2008 in the Department of Polymer and Fiber Engineering. His research interests include the microwave approach to ultrafast production of nanomaterials, mechanism study of polymeric material self-assembly using nano-seeding approach, chemical/electrochemical sensors, and polymer-metal nanocomposites. Currently, he is an Associate Professor in the Department of Chemical Engineering, Auburn University.
Rights and permissions
About this article
Cite this article
Sarwar, S., Nautiyal, A., Cook, J. et al. Facile microwave approach towards high performance MoS2/graphene nanocomposite for hydrogen evolution reaction. Sci. China Mater. 63, 62–74 (2020). https://doi.org/10.1007/s40843-019-9555-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-019-9555-0