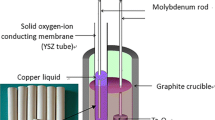
Abstract
The electro-calciothermic reduction of tantalum sulfide (TaS2) was applied to open a new pathway for producing high-purity Ta powders applicable for tantalum electrolytic capacitors. The supplied electric charge was varied to electrochemically reduce the sulfide at 900 °C using Ca in molten CaCl2−xCaS (x = 0.1, 0.5 mol%) salts. The cleaner TaS2 could also be prepared via the highly efficient carbo-sulfidation of Ta2O5 using S2 gas. The plate-like Ta particles with internal voids and coral structures were obtained through the electrochemical reduction of the sulfides. Sulfur removal was achieved rapidly from TaS2. Ta powder with 0.08 mass% O and 0.01 mass% S was successfully produced from Ta2O5 via clean TaS2. It was obtained by increasing the electric charge to two times higher than that required to generate the stoichiometric amount of Ca in the CaCl2 melt. Therefore, the electrometallurgical reduction of TaS2 could be a promising, cost-effective, and sustainable approach for producing high-purity Ta powders.
Graphical Abstract









Similar content being viewed by others
References
Buckman RW (2000) New applications for tantalum and tantalum alloys. JOM 52:40–41
de Brito RA, Medeiros FFP, Gomes UU, Costa FA, Silva AGP, Alves C (2008) Production of tantalum by aluminothermic reduction in plasma reactor. Int J Refract Met Hard Mater 26:433–437
Baba M, Suzuki RO (2005) Dielectric properties of tantalum powder with broccoli-like morphology. J Alloys Compd 392:225–230
Baba M, Ono Y, Suzuki RO (2005) Tantalum and niobium powder preparation from their oxides by calciothermic reduction in the molten CaCl2. J Phys Chem Solids 66:466–470
Suzuki RO, Baba M, Ono Y, Yamamoto K (2005) Formation of broccoli-like morphology of tantalum powder. J Alloys Compd 389:310–316
Niu B, Chen Z, Xu Z (2017) Recovery of tantalum from waste tantalum capacitors by supercritical water treatment. ACS Sustain Chem 5:4421–4428
Zhou Y-L, Niinomi M, Akahori T, Nakai M, Fukui H (2007) Comparison of various properties between titanium-tantalum alloy and pure titanium for biomedical applications. Mater Trans 48:380–384
Huang S, Sing SL, de Looze G, Wilson R, Yeong WY (2020) Laser powder bed fusion of titanium-tantalum alloys: compositions and designs for biomedical applications. J Mech Behav Biomed Mater 108:103775
Balagna C, Faga MG, Spriano S (2011) Tantalum-based thin film coatings for wear resistant arthroprostheses. J Nanosci Nanotechnol 11:8994–9002
Manso AP, Marzo FF, Garicano X, Alegre C, Lozano A, Barreras F (2020) Corrosion behavior of tantalum coatings on AISI 316L stainless steel substrate for bipolar plates of PEM fuel cells. Int J Hydrog Energy 45:20679–20691
Rosenberg H, Bahri O, Wang G, LaRue W (2005) Tantalum sputtering target and method of manufacture, United States Patent, US 6,955,938 B2
Cardonne SM, Kumar P, Michaluk CA, Schwartz HD (1995) Tantalum and its alloys. Int J Refract Met Hard Mater 13:187–194
Köck W, Paschen P (1989) Tantalum—processing, properties and applications. JOM 41:33–39
Okabe TH, Sato N, Mitsuda Y, Ono S (2003) Production of tantalum powder by magnesiothermic reduction of feed preform. Mater Trans 44:2646–2653
Mineta K, Okabe TH (2005) Development of a recycling process for tantalum from capacitor scraps. J Phys Chem Solids 66:318–321
Reynolds C (2016) Tantalum capacitor technology options for high temperature and harsh environment applications. Addit Conf (Device Pakag, HiTEC, HiTEN, and CICMT). https://doi.org/10.4071/2016-HITEC-299
Suzuki RO, Ishii R, Nishiyama T, Iijima H (2003) Powdered tantalum, niobium, production process thereof and porous sintered body and solid electrolytic capacitor using the powdered tantalum or niobium, United States Patent, US 2003/0206390 A1
Yang G, Zheng A, Cheng Y, Ma Y (2016) Method for preparing tantalum powder of capacitor grade with high nitrogen content, tantalum powder of capacitor grade prepared by the process, and an anode and a capacitor made of the tantalum powder, United States Patent, 0059319 A1
Yuan B, Okabe TH (2007) Production of fine tantalum powder by electrochemical method. Mater Trans 48:2687–2694
Hwang S-M, Wang J-P, Lee D-W (2019) Extraction of tantalum powder via the magnesium reduction of tantalum pentoxide. Metals 9:205
Barnett R, Kilby KT, Fray DJ (2009) Reduction of tantalum pentoxide using graphite and tin-oxide-based anodes via the FFC-Cambridge Process. Metall Mater Trans B 40:150–157
Yan XY, Fray DJ (2011) Using electro-deoxidation to synthesize niobium sponge from solid Nb2O5 in alkali–alkaline-earth metal chloride melts. J Mater Res 18:346–356
Barnett RP, Fray DJ (2013) Reaction of tantalum oxide with calcium chloride–calcium oxide melts. J Mater Sci 48:2581–2589
Garg SP, Krishnamurthy N, Awasthi A, Venkatraman M (1996) The O-Ta (oxygen-tantalum) system. J Phase Equilib 17:63–77
Suzuki N, Tanaka M, Noguchi H, Natsui S, Kikuchi T, Suzuki RO (2017) Calcium reduction of TiS2 in CaCl2 melt. Mater Trans 58:367–370
Ahmadi E, Suzuki RO (2020) An innovative process for production of Ti metal powder via TiSx from TiN. Metall Mater Trans B 51:140–148
Ahmadi E, Suzuki RO, Kikuchi T, Kaneko T, Yashima Y (2020) Towards a sustainable technology for production of extra-pure Ti metal: electrolysis of sulfurized Ti(C, N) in molten CaCl2. Int J Miner Metall Mater 27:1635–1643
Ono K, Suzuki RO (2002) A new concept for producing Ti sponge: calciothermic reduction. JOM 54:59–61
Suzuki RO, Yashima Y, Suzuki N, Ahmadi E, Natsui S, Kikuchi T (2020) Titanium production via titanium sulfide. MATEC Web Conf 321:07003
Kaneko T, Yashima Y, Ahmadi E, Natsui S, Suzuki RO (2020) Synthesis of Sc sulfides by CS2 sulfurization. J Solid State Chem 285:121268
Ahmadi E, Yashima Y, Suzuki RO, Rezan SA (2018) Formation of titanium sulfide from titanium oxycarbonitride by CS2 gas. Metall Mater Trans B 49:1808–1821
Tsai C-H, Lee W-J, Chen C-Y, Liao W-T, Shih M (2002) Formation of solid sulfur by decomposition of carbon disulfide in the oxygen-lean cold plasma environment. Ind Eng Chem Res 41:1412–1418
Roine A, Kobylin P (2014) Outokumpu HSC Chemistry for Windows, Chemical reaction and equilibrium software with extensive thermochemical database. Outotec Research Center, Pori, Finland, HSC Ver. 8.08
Wu C, Tan M, Ye G, Fray DJ, ** X (2019) High-efficiency preparation of titanium through electrolysis of carbo-sulfurized titanium dioxide. ACS Sustain Chem Eng 7:8340–8346
Matsuzaki T, Suzuki RO, Natsui S, Kikuchi T, Ueda M (2019) Solubility of CaS in molten CaCl2. Mater Trans 60:386–390
Suzuki RO, Natsui S, Kikuchi T (2020) OS process: calciothermic reduction of TiO2 via CaO electrolysis in molten CaCl2. In: Fang ZZ, Froes FH, Zhang Y (eds) Extractive metallurgy of titanium: conventional and recent advances in extraction and production of titanium metal, 1st edn. Elsevier, Amsterdam, pp 287–313
Ahmadi E, Suzuki RO, Kaneko T, Kikuchi T (2020) A sustainable approach for producing Ti and TiS2 from TiC. Metall Mater Trans B 52:77–87. https://doi.org/10.1007/s11663-020-01988-5
Spijkerman A, de Boer JL, Meetsma A, Wiegers GA, Smaalen SV (1997) X-ray crystal-structure refinement of the nearly commensurate phase of 1T-TaS2 in (3+2)-dimensional superspace. Phys Rev B 56:13757–13767
Luxa J, Mazánek V, Pumera M, Lazar P, Sedmidubský D, Callisti M, Polcar T, Sofer Z (2017) 2H→1T phase engineering of layered tantalum disulfides in electrocatalysis: oxygen reduction reaction. Chem Eur J 23:8082–8091
Sohn HY, Szekely J (1972) A structural model for gas-solid reactions with a moving boundary—III: a general dimensionless representation of the irreversible reaction between a porous solid and a reactant gas. Chem Eng Sci 27:763–778
Franzen HF, Smeggil JG (1969) The crystal structure of Ta2S. Acta Cryst B25:1736–1741
Franzen HF, Smeggil JG (1970) The crystal structure of Ta6S. Acta Cryst B26:125–129
Wada H, Onoda M (1989) On the preparation and structure of the compound Ta3S1.8. Mater Res Bull 24:191–196
Haraguchi Y, Shibuya R, Natsui S, Kikuchi T, Suzuki RO (2019) Gas generation reactions during TiO2 reduction using molten salt. J Japan Inst Met Mater 83:441–448
Suzuki RO, Ono K, Teranuma K (2003) Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B 34:287–295
Gill J (2020) Technical information, basic tantalum capacitor technology, AVX Ltd., Tantalum Division Paignton, England. https://www.yumpu.com/en/document/read/42806826/basic-tantalum-capacitor-technology-avx. Accessed 10 Nov 2020
Hunter MA (1953) Early history of titanium. JOM 5:130–132
Sahu SK, Chmielowiec B, Allanore A (2017) Electrolytic extraction of copper, molybdenum and rhenium from molten sulfide electrolyte. Electrochim Acta 243:382–389
Okamoto H (1998) C-Ta (carbon-tantalum). J Phase Equilib 19:88
Vaughan DA, Stewart OM, Schwartz CM (1960) Determination of interstitial solid-solubility limit in tantalum and identification of the precipitate phases. Battelle Memorial Institute, 15th edn., UC-25 Metallurgy and Ceramics, TID-4500, Report No. BMI-1472.
Freeman Y (2018) Tantalum and niobium-based capacitors: science, technology, and applications. Springer, Cham. https://doi.org/10.1007/978-3-319-67870-2
Gebhardt E, Seghezzi HD (1959) Investigation in system Ta–O. II. Reaction and equilibrium between mixed crystal andoxide phases. Z Metallkd 50:521
Acknowledgements
This work was financially supported by the Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship (No. P18054), Grants-in-Aid for Scientific Research (KAKENHI Grant Nos 18F18054 and 17H03434) and the Japan Mining Industry Association. The kind support from JSPS and the International Affairs Office of the Faculty of Engineering, Hokkaido University, are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. We do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
The contributing editor for this article was Hongmin Zhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmadi, E., Suzuki, R.O. Tantalum Metal Production Through High-Efficiency Electrochemical Reduction of TaS2 in Molten CaCl2. J. Sustain. Metall. 7, 437–447 (2021). https://doi.org/10.1007/s40831-021-00347-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00347-1