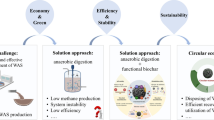
Abstract
Purpose of Review
This review intends to recapitulate the pretreatment measures of kitchen waste and kitchen wastewater (KWAKWW). Furthermore, this review also separately summarizes the ascendancy of using bacteria, microalgae and microalgae-bacteria consortia to treat KWAKWW, and corresponding emerging reinforcement strategies.
Recent Findings
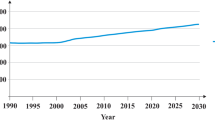
Tremendous amount of KWAKWW are annually generated in the whole world. Wherein roughly 1.3 billion tons of kitchen waste (KW) are dumped and which were forecasted that would increase to about 2.5 billion tons by 2025. In addition, KWAKWW have the characteristics of high content of refractory organic matter (e.g., oil and cellulose), water (commonly outstrip 70%), and salt, which is difficult for bacteria or microalgae to treat. Consequently, it is essential to perform efficacious pretreatment measures to boost the efficiency of post-treatment. Utilizing bacteria, microalgae, and microalgae-bacteria consortia to treat KWAKWW is considered an efficient strategy due to ascendancy of puissant deep purification ability, excellent resource appreciation effect, and low operation costs. For instance, bacteria could produce leastways four kinds of products through KWAKWW; multiple studies indicated that microalgae generally could remove exceed 70% of nutrients of KWAKWW; one research manifested that microalgae-bacteria consortia retrenched 46% of the demand about dissolved oxygen (DO). Nevertheless, the above microbial treatment systems still have some inherent drawbacks such as poor impact resistance. Fortunately, metabolic engineering and other strengthen strategies can efficaciously upgrade the nutrient removal and resource utilization performance of bacteria, microalgae, and microalgae-bacteria consortia. For example, one research shown that the 1-butanol productivity of original bacteria remarkably increased by 93.48–171.74% draw support from metabolic engineering.
Summary
A total of 221 papers related to the content of this review were searched through Web of Science (http://apps.webofknowledge.com). What is more, specific data that emerged on this review were all extracted from the above-searched papers. The mechanisms and effect of hydrothermal carbonization (HTC) and other four pretreatment measures are introduced by this review in detail. The preponderance of utilizing bacteria, microalgae, and microalgae-bacteria consortia to treat KWAKWW are comprehensively evaluated mainly from the perspectives of nutrient purification and resource utilization. Four state-of-the-art strengthen strategies like machine learning are then introduced. Finally, the current challenges in KWAKWW treatment are summarized from five aspects, and future concrete improvement directions are also provided. Overall, this review outlines the state-of-the-art research progress of KWAKWW treatment by bacteria and microalgae and tenders corresponding implementation schemes for improving KWAKWW treatment effect.





Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Huang J, Pan Y, Liu L, Liang J, Wu L, Zhu H, Zhang P. High salinity slowed organic acid production from acidogenic fermentation of kitchen wastewater by sha** functional bacterial community. J Environ Manag. 2022;310:114765. https://doi.org/10.1016/j.jenvman.2022.114765.
• Elgarahy AM, Eloffy MG, Alengebawy A, El-Sherif DM, Gaballah MS, Elwakeel KZ, El-Qelish M. Sustainable management of food waste; pre-treatment strategies, techno-economic assessment, bibliometric analysis, and potential utilizations: a systematic review. Environ Res. 2023;225:115558. https://doi.org/10.1016/j.envres.2023.115558. This review detailed and summarized the characteristics, composition, and pretreatment measures of KWAKWW and introduced different valuable products derived from KWAKWW.
• Liu X, Shen J, Guo Y, Wang S, Chen B, Luo L, Zhang H. Technical progress and perspective on the thermochemical conversion of kitchen waste and relevant applications: a comprehensive review. Fuel. 2023;331:125803. https://doi.org/10.1016/j.fuel.2022.125803. This review explained the specific conversion pathways of principal components in KWAKWW during the HTC process.
Nazia S, Jegatheesan V, Bhargava SK, Sundergopal S. Microbial fuel cell–aided processing of kitchen wastewater using high-performance nanocomposite membrane. J Environ Eng. 2020;8:146. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001717.
• Ahmad I, Abdullah N, Koji I, Yuzir A, Mohamad SE, Show PL, Cheah WY, Kho KS. The role of restaurant wastewater for producing bioenergy towards a circular bioeconomy: a review on composition, environmental impacts, and sustainable integrated management. Environ Res. 2022;214:113854. https://doi.org/10.1016/j.envres.2022.113854. This review summarized the characteristics and disservices of KWW.
Esteban-Lustres R, Torres MD, Piñeiro B, Enjamio C, Domínguez H. Intensification and biorefinery approaches for the valorisation of kitchen wastes – a review. Bioresour Technol. 2022;360:127652. https://doi.org/10.1016/j.biortech.2022.127652.
Cai L, Guo H, Zheng G, Wang X, Wang K. Metagenomic analysis reveals the microbial degradation mechanism during kitchen waste biodrying. Chemosphere. 2022;307:135862. https://doi.org/10.1016/j.chemosphere.2022.135862.
Guleria S, Singh H, Sharma V, Bhardwaj N, Arya SK, Puri S, Khatri M. Polyhydroxyalkanoates production from domestic waste feedstock: a sustainable approach towards bio-economy. J Clean Prod. 2022;340:130661. https://doi.org/10.1016/j.jclepro.2022.130661.
Kumar Y, Kaur S, Kheto A, Munshi M, Sarkar A, Pandey HO, Tarafdar A, Sirohi R. Cultivation of microalgae on food waste: recent advances and way forward. Bioresour Technol. 2022;363:127834. https://doi.org/10.1016/j.biortech.2022.127834.
•• Wang Z, Chu Y, Chang H, **e P, Zhang C, Li F, Ho S-H. Advanced insights on removal of antibiotics by microalgae-bacteria consortia: a state-of-the-art review and emerging prospects. Chemosphere. 2022;307(Part 4):136117. https://doi.org/10.1016/j.chemosphere.2022.136117. This review summarized interaction mechanisms between microalgae and bacteria and introduced some novel treatment systems based on microalgae-bacteria consortia.
Oruganti RK, Katam K, Show PL, Gadhamshetty V, Upadhyayula VKK, Bhattacharyya D. A comprehensive review on the use of algal-bacterial systems for wastewater treatment with emphasis on nutrient and micropollutant removal. Bioengineered. 2022;13(4):10412–53. https://doi.org/10.1080/21655979.2022.2056823.
Palansooriya KN, Dissanayake PD, Igalavithana AD, Tang RG, Cai YJ, Chang SX. Converting food waste into soil amendments for improving soil sustainability and crop productivity: a review. Sci Total Environ. 2023;881:163311. https://doi.org/10.1016/j.scitotenv.2023.163311.
**e T, Zhang Z, Zhang D, Wei C, Lin Y, Feng R, Nan J, Feng Y. Effect of hydrothermal pretreatment and compound microbial agents on compost maturity and gaseous emissions during aerobic composting of kitchen waste. Sci Total Environ. 2022;854:158712. https://doi.org/10.1016/j.scitotenv.2022.158712.
• Chew KW, Chia SR, Show PL, Ling TC, Arya SS, Chang J-S. Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris. Bioresour Technol. 2018;267:356–62. https://doi.org/10.1016/j.biortech.2018.07.069. This article explored the effect of dilution and composting these two KWAKWW pretreatment measures to the treatment ability of microalgae and, besides, explored the high-value addition substance accumulation performance of microalgae under different pretreatment conditions.
Fernandes F, Silkina A, Fuentes-Grünewald C, Wood EE, Ndovela VLS, OatleyRadcliffe DL, Lovitt RW, Llewellyn CA. Valorising nutrient-rich digestate: dilution, settlement and membrane filtration processing for optimization as a waste-based media for microalgal cultivation. Waste Manag. 2020;118:197–208. https://doi.org/10.1016/J.WASMAN.2020.08.037.
Sforza E, Simionato D, Giacometti GM, Bertucco A, Morosinotto T. Adjusted light and dark cycles can optimize photosynthetic efficiency in algae growing in photobioreactors. PLoS ONE. 2012;7:e38975. https://doi.org/10.1371/journal.pone.0038975.
Hafid HS, Shah UKM, Baharuddin AS, Ariff AB. Feasibility of using kitchen waste as future substrate for bioethanol production: a review. Renew Sustain Energy Rev. 2017;74:671–86. https://doi.org/10.1016/j.rser.2017.02.071.
Li P, Zeng Y, **e Y, Li X, Kang Y, Wang Y, **e T, Zhang Y. Effect of pretreatment on the enzymatic hydrolysis of kitchen waste for xanthan production. Bioresour Technol. 2017;223:84–90. https://doi.org/10.1016/j.biortech.2016.10.035.
Chavan S, Yadav B, Tyagi RD, Wong JWC, Drogui P. Trends and challenges in the valorization of kitchen waste to polyhydroxyalkanoates. Bioresour Technol. 2023;369:128323. https://doi.org/10.1016/j.biortech.2022.128323.
Li L-H, Li Y-L, Hong Y. New insights into the microalgal culture using kitchen waste: enzyme pretreatment and mixed microalgae self-flocculation. Biochem Eng J. 2023;195:108904. https://doi.org/10.1016/j.bej.2023.108904.
Gao X, Xu Z, Li Y, Zhang L, Li G, Nghiem LD, Luo W. Bacterial dynamics for gaseous emission and humification in bio-augmented composting of kitchen waste. Sci Total Environ. 2021;801:149640. https://doi.org/10.1016/j.scitotenv.2021.149640.
Zhou Y, Engler N, Nelles M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour Technol. 2018;260:404–12. https://doi.org/10.1016/j.biortech.2018.03.102.
Ferrentino R, Merzari F, Grigolini E, Fiori L, Andreottola G. Hydrothermal carbonization liquor as external carbon supplement to improve biological denitrification in wastewater treatment. J Water Process Eng. 2021;44:102360. https://doi.org/10.1016/j.jwpe.2021.102360.
Hu Z, Shi X, Jiang H. Correlating the chemical properties and bioavailability of dissolved organic matter released from hydrochar of walnut shells. Chemosphere. 2021;275:130003. https://doi.org/10.1016/j.chemosphere.2021.130003.
Tarhan SZ, Koçer AT, Özçimen D, Gökalp İ. Cultivation of green microalgae by recovering aqueous nutrients in hydrothermal carbonization process water of biomass wastes. J Water Proc Eng. 2021;40:101783. https://doi.org/10.1016/j.jwpe.2020.101783.
Patel A, Mahboubi A, Horváth IS, Taherzadeh MJ, Rova U, Christakopoulos P, Matsakas L. Volatile fatty acids (VFAs) generated by anaerobic digestion serve as feedstock for freshwater and marine oleaginous microorganisms to produce biodiesel and added-value compounds. Front Microbiol. 2021;12:1–17. https://doi.org/10.3389/fmicb.2021.614612.
Al-Mallahi J, Ishii K. Attempts to alleviate inhibitory factors of anaerobic digestate for enhanced microalgae cultivation and nutrients removal: a review. J Environ Manage. 2022;304:114266. https://doi.org/10.1016/j.jenvman.2021.114266.
Wu KC, Yau YH. Sze ETP. Application of anaerobic bacterial ammonification pretreatment to microalgal food waste leachate cultivation and biofuel production. Mar Pollut Bull. 2020;153:111007. https://doi.org/10.1016/j.marpolbul.2020.111007.
Chong CC, Cheng YW, Ishak S, Lam MK, Lim JW, Tan IS, et al. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: a way forward through waste valorization approach. Sci Total Environ. 2022;803:150070. https://doi.org/10.1016/j.scitotenv.2021.150070.
Raj T, Chandrasekhar K, Morya R, Kumar Pandey A, Jung JH, Kumar D, Singhania RR, Kim SH. Critical challenges and technological breakthroughs in food waste hydrolysis and detoxification for fuels and chemicals production. Bioresour Technol. 2022;360:127512. https://doi.org/10.1016/j.biortech.2022.127512.
Dhinam S, Mukherjee G. Present scenario and future scope of food waste to biofuel production. J Food Process Eng. 2021;44(2):13594. https://doi.org/10.1111/jfpe.13594.
Melikoglu M. Reutilisation of food wastes for generating fuels and value added products: a global review. Environ Technol Innov. 2020;19:101040. https://doi.org/10.1016/j.eti.2020.101040.
Wang C, Wu M, Peng C, Yan F, Jia Y, Li X, Li M, Wu B, Xu H, Qiu Z. Bacterial dynamics and functions driven by a novel microbial agent to promote kitchen waste composting and reduce environmental burden. J Clean Prod. 2022;337:130491. https://doi.org/10.1016/j.jclepro.2022.130491.
Wang S, Xu C, Song L, Zhang J. Anaerobic digestion of food waste and its microbial consortia: a historical review and future perspectives. Int J Environ Res Public Health. 2022;19(15):9519. https://doi.org/10.3390/ijerph19159519.
**n L, Qin Y, Lou T, Xu X, Wang H, Mei Q, Wu W. Rapid start-up and humification of kitchen waste composting by an innovative biodrying-enhanced process. Chem Eng J. 2023;452:139459. https://doi.org/10.1016/j.cej.2022.139459.
Xu Z, Qi C, Zhang L, Ma Y, Li G, Nghiem LD, Luo W. Regulating bacterial dynamics by lime addition to enhance kitchen waste composting. Bioresour Technol. 2021;341:125749. https://doi.org/10.1016/j.biortech.2021.125749.
Ajay CM, Mohan S, Dinesha P. Decentralized energy from portable biogas digesters using domestic kitchen waste: a review. Waste Manag. 2021;125:10–26. https://doi.org/10.1016/j.wasman.2021.02.031.
Yan M, Liu Y, Song Y, Xu A, Zhu G, Jiang J. Comprehensive experimental study on energy conversion of household kitchen waste via integrated hydrothermal carbonization and supercritical water gasification. Energy. 2022;242:123054. https://doi.org/10.1016/j.energy.2021.123054.
Deng H, Ren H, Fan J, Zhao K, Hu C, Qu J. Membrane fouling mitigation by coagulation and electrostatic repulsion using an electro-AnMBR in kitchen wastewater treatment. Water Res. 2022;222:118883. https://doi.org/10.1016/j.watres.2022.118883.
Duan N, Khoshnevisan B, Lin C, Liu Z, Liu H. Life cycle assessment of anaerobic digestion of pig manure coupled with different digestate treatment technologies. Environ Int. 2020;137:105522. https://doi.org/10.1016/j.envint.2020.105522.
** C, Sun S, Yang D, Sheng W, Ma Y, He W, Li G. Anaerobic digestion: an alternative resource treatment option for food waste in China. Sci Total Environ. 2021;779:146397. https://doi.org/10.1016/j.scitotenv.2021.146397.
Li Y, ** Y, Borrion A, Li H. Current status of food waste generation and management in China. Bioresour Technol. 2019;273:654–65. https://doi.org/10.1016/j.biortech.2018.10.083.
Meng Q, Liu H, Zhang H, Xu S, Lichtfouse E, Yun Y. Anaerobic digestion and recycling of kitchen waste: a review. Environ Chem Lett. 2022;20(3):1745–62. https://doi.org/10.1007/s10311-022-01408-x.
Narisetty V, Adlakha N, Kumar Singh N, Dalei SK, Prabhu AA, Nagarajan S, Naresh Kumar A, Amruthraj Nagoth J, Kumar G, Singh V, Kumar V. Integrated biorefineries for repurposing of food wastes into value-added products. Bioresour Technol. 2022;363:127856. https://doi.org/10.1016/j.biortech.2022.127856.
Zhang X, Zhang D, Chu S, Khalid M, Wang R, Chi Y, Duan X, Yang X, Zhou P. Employing salt-tolerant bacteria Serratia marcescens subsp. SLS for biodegradation of oily kitchen waste. Chemosphere. 2023;329:138655. https://doi.org/10.1016/j.chemosphere.2023.138655.
Ke X, Sun JC, Liu C, Ying JM, Zou SP, Xue YP, Zheng YG. Fed-in-situ biological reduction treatment of food waste via high-temperature-resistant oil degrading microbial consortium. Bioresour Technol. 2021;340:125635. https://doi.org/10.1016/j.biortech.2021.125635.
Wang C, Li J, Fang W, Chen W, Zou M, Li X, Qiu Z, Xu H. Lipid degrading microbe consortium driving micro-ecological evolvement of activated sludge for cooking wastewater treatment. Sci Total Environ. 2022;804:150071. https://doi.org/10.1016/J.SCITOTENV.2021.150071.
Taipabu MI, Viswanathan K, Wu W, Nagy ZK. Production of renewable fuels and chemicals from fats, oils, and grease (FOG) using homogeneous and heterogeneous catalysts: design, validation, and optimization. Chem Eng J. 2021;424:130199. https://doi.org/10.1016/j.cej.2021.130199.
Ke X, Hua X, Sun JC, Zheng RC, Zheng YG. Synergetic degradation of waste oil by constructed bacterial consortium for rapid in-situ reduction of kitchen waste. J Biosci Bioeng. 2021;131(4):412–9. https://doi.org/10.1016/j.jbiosc.2020.12.005.
Kumar P, Sharma R, Ray S, Mehariya S, Patel SKS, Lee J-K, Kalia VC. Dark fermentative bioconversion of glycerol to hydrogen by bacillus thuringiensis. Bioresour Technol. 2015;182:383–8. https://doi.org/10.1016/j.biortech.2015.01.138.
Metsoviti M, Zeng A-P, Koutinas AA, Papanikolaou S. Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J Biotechnol. 2013;163:408–18. https://doi.org/10.1016/j.jbiotec.2012.11.018.
Ben Ayed H, Jemil N, Maalej H, Bayoudh A, Hmidet N, Nasri M. Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. Int Biodeterior Biodegradation. 2015;99:8–14. https://doi.org/10.1016/j.ibiod.2014.12.009.
Cao M-K, Guo H-T, Zheng G-D, Chen T-B, Cai L. Microbial succession and degradation during kitchen waste biodrying, highlighting the thermophilic phase. Bioresour Technol. 2021;326:124762. https://doi.org/10.1016/j.biortech.2021.124762.
Pham VHT, Kim J, Shim J, Chang S, Chung W. Coconut mesocarp-based lignocellulosic waste as a substrate for cellulase production from high promising multienzyme-producing Bacillus amyloliquefaciens FW2 without pretreatments. Microorganisms. 2022;10:327. https://doi.org/10.3390/microorganisms10020327.
Li F, Ghanizadeh H, Cui G, Liu J, Miao S, Liu C, Song W, Chen X, Cheng M, Wang P, Zhang Y, Wang A. Microbiome - based agents can optimize composting of agricultural wastes by modifying microbial communities. Bioresour Technol. 2023;374:128765. https://doi.org/10.1016/j.biortech.2023.128765.
Parwin R, Paul KK. Overview of applications of kitchen wastewater and its treatment. J Hazard Toxic Radio Waste. 2020;24:04019041. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000482.
Sindhu R, Manju A, Mohan P, Rajesh RO, Madhavan A, Arun KB, Hazeena SH, Mohandas A, Rajamani SP, Puthiyamadam A, Binod P, Reshmy R. Valorization of food and kitchen waste: an integrated strategy adopted for the production of poly-3-hydroxybutyrate, bioethanol, pectinase and 2, 3-butanediol. Bioresour Technol. 2020;310:123515. https://doi.org/10.1016/j.biortech.2020.123515.
Hallenbeck PC, Abo-Hashesh M, Ghosh D. Strategies for improving biological hydrogen production. Bioresour Technol. 2012;110:1–9. https://doi.org/10.1016/j.biortech.2012.01.103.
Mohanakrishna G, Sneha NP, Rafi SM, Sarkar O. Dark fermentative hydrogen production: potential of food waste as future energy needs. Sci Total Environ. 2023;888:163801. https://doi.org/10.1016/j.scitotenv.2023.163801.
Slezak R, Grzelak J, Krzystek L, Ledakowicz S. The effect of initial organic load of the kitchen waste on the production of VFA and H2 in dark fermentation. Waste Manage. 2017;68:610–7. https://doi.org/10.1016/j.wasman.2017.06.024.
Hai T, Mishra P, Zain JM, Saini K, Kumar NM, Ab WZ. Co-digestion of domestic kitchen food waste and palm oil mill effluent for biohydrogen production. Sustainable Energy Technol Assess. 2023;55:102965. https://doi.org/10.1016/j.seta.2022.102965.
Hees T, Zhong F, Stürzel M, Mülhaupt R. Tailoring hydrocarbon polymers and all-hydrocarbon composites for circular economy. Macromol Rapid Commun. 2019;40(1):1800608. https://doi.org/10.1002/marc.201800608.
Rawoof SAA, Kumar PS, Devaraj K, Devaraj T, Subramanian S. Enhancement of lactic acid production from food waste through simultaneous saccharification and fermentation using selective microbial strains. Biomass Convers Biorefinery. 2022;12:5947–58. https://doi.org/10.1007/s13399-020-00998-2.
Swetha TA, Ananthi V, Bora A, Sengottuvelan N, Ponnuchamy K, Muthusamy G, Arun A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste - its applications and degradation. Int J of Biol Macromol. 2023;234:123703. https://doi.org/10.1016/j.ijbiomac.2023.123703.
Kumar G, Ponnusamy VK, Bhosale RR, Shobana S, Yoon JJ, Bhatia SK, Rajesh Banu J, Kim SH. A review on the conversion of volatile fatty acids to polyhydroxyalkanoates using dark fermentative effluents from hydrogen production. Bioresour Technol. 2019;287:121427. https://doi.org/10.1016/j.biortech.2019.121427.
Rao A, Haque S, El-Enshasy HA, Singh V, Mishra BN. RSM–GA based optimization of bacterial PHA production and in silico modulation of citrate synthase for enhancing PHA production. Biomolecules. 2019;9(12):872. https://doi.org/10.3390/biom9120872.
Chhandama MVL, Chetia AC, Satyan KB, Ao S, Ruatpuia JV, Rokhum SL. Valorisation of food waste to sustainable energy and other value-added products: a review. Bioresour Technol Rep. 2022;17:100945. https://doi.org/10.1016/j.biteb.2022.100945.
Panahi HKS, Dehhaghi M, Guillemin GJ, Lam SS, Aghbashlo M, Tabatabaei M. Bioethanol production from food wastes rich in carbohydrates. Curr Opin Food Sci. 2022;43:71–81. https://doi.org/10.1016/j.cofs.2021.11.001.
Sarkar D, Gupta K, Poddar K, Biswas R, Sarkar A. Direct conversion of fruit waste to ethanol using marine bacterial strain Citrobacter sp. E4. Process Saf Environ Prot. 2019;128:203–10. https://doi.org/10.1016/j.psep.2019.05.051.
Xue C, Zhao XQ, Liu CG, Chen LJ, Bai FW. Prospective and development of butanol as an advanced biofuel. Biotechnol Adv. 2013;31(8):1575–84. https://doi.org/10.1016/j.biotechadv.2013.08.004.
Li J, Du Y, Bao T, Dong J, Lin M, Shim H, Yang S-T. n-Butanol production from lignocellulosic biomass hydrolysates without detoxification by Clostridium tyrobutyricum Δack-adhE2 in a fibrous-bed bioreactor. Bioresour Technol. 2019;289:121749. https://doi.org/10.1016/j.biortech.2019.121749.
Zhang J, Zong W, Hong W, Zhang ZT, Wang Y. Exploiting endogenous CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab Eng. 2018;47:49–59. https://doi.org/10.1016/j.biombioe.2018.05.012.
Chen H, Shen H, Su H, Chen H, Tan F, Lin J. High-efficiency bioconversion of kitchen garbage to biobutanol using an enzymatic cocktail procedure. Bioresour Technol. 2017;245:1110–21. https://doi.org/10.1016/j.biortech.2017.09.056.
Saady NMC. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: unresolved challenge. Int J Hydrog Energy. 2013;38:13172–91. https://doi.org/10.1016/j.ijhydene.2013.07.122.
Tawfik A, El-Qelish M, Salem A. Efficient anaerobic co-digestion of municipal food waste and kitchen wastewater for bio-hydrogen production. Int J Green Energy. 2015;12:1301–8. https://doi.org/10.1080/15435075.2014.909357.
Merli G, Becci A, Amato A, Beolchini F. Acetic acid bioproduction: the technological innovation change. Sci Total Environ. 2021;798:149292. https://doi.org/10.1016/j.scitotenv.2021.149292.
Upadhyay A, Kovalev AA, Zhuravleva EA, Pareek N, Vivekanand V. Enhanced production of acetic acid through bioprocess optimization employing response surface methodology and artificial neural network. Bioresour Technol. 2023;376:128930. https://doi.org/10.1016/j.biortech.2023.128930.
Li Y, He D, Niu D, Zhao Y. Acetic acid production from food wastes using yeast and acetic acid bacteria micro-aerobic fermentation. Bioprocess Biosyst Eng. 2015;38:863–9. https://doi.org/10.1007/s00449-014-1329-8.
Wang J, Zeng A, Yuan W. Succinic acid fermentation from agricultural wastes: the producing microorganisms and their engineering strategies. Curr Opin Environ Sci Heal. 2022;25:100313. https://doi.org/10.1016/j.coesh.2021.100313.
Ahn JH, Jang YS, Lee SY. Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol. 2016;42:54–66. https://doi.org/10.1016/j.copbio.2016.02.034.
Kuglarz M, Angelidaki I. Succinic Production from source-separated kitchen biowaste in a biorefinery concept: focusing on alternative carbon dioxide source for fermentation processes. Fermentation. 2023;9:259. https://doi.org/10.3390/fermentation9030259.
Babaei M, Tsapekos P, Alvarado-Morales M, Hosseini M, Ebrahimi S, Niaei A, Angelidaki I. Valorization of organic waste with simultaneous biogas upgrading for the production of succinic acid. Biochem Eng J. 2019;147:136–45. https://doi.org/10.1016/j.bej.2019.04.012.
Chen Y, Zhang X, Chen Y. Propionic acid-rich fermentation (PARF) production from organic wastes: a review. Bioresour Technol. 2021;339:125569. https://doi.org/10.1016/j.biortech.2021.125569.
Strazzera G, Battista F, Garcia NH, Frison N, Bolzonella D. Volatile fatty acids production from food wastes for biorefinery platforms: a review. J Environ Manage. 2018;226:278–88. https://doi.org/10.1016/j.jenvman.2018.08.039.
Zheng Y, Wang P, Yang X, Zhao L, Ren L, Li J. Metagenomics insight into bioaugmentation mechanism of Propionibacterium acidipropionici during anaerobic acidification of kitchen waste. Bioresour Technol. 2022;362:127843. https://doi.org/10.1016/j.biortech.2022.127843.
Kim D-H, Kim S-H, Jung K-W, Kim M-S, Shin H-S. Effect of initial pH independent of operational pH on hydrogen fermentation of food waste. Bioresour Technol. 2011;102(18):8646–52. https://doi.org/10.1016/j.biortech.2011.03.030.
Zhang L, Li J, Ban Q, He J, Jha AK. Metabolic pathways of hydrogen production in fermentative acidogenic microflora. J Microbiol Biotechnol. 2012;22:668–73. https://doi.org/10.4014/jmb.1110.10076.
Jung JH, Sim YB, Baik JH, Park JH, Kim SH. High-rate mesophilic hydrogen production from food waste using hybrid immobilized microbiome. Bioresour Technol. 2021;320:124279. https://doi.org/10.1016/j.biortech.2020.124279.
Lee D-Y, Xu K-Q, Kobayashi T, Li Y-Y, Inamori Y. Effect of organic loading rate on continuous hydrogen production from food waste in submerged anaerobic membrane bioreactor. Int J Hydrog Energy. 2014;39:16863–71. https://doi.org/10.1016/j.ijhydene.2014.08.022.
Kanchanasuta S, Prommeenate P, Boonapatcharone N, Pisutpaisal N. Stability of Clostridium butyricum in biohydrogen production from non-sterile food waste. Int J Hydrog Energy. 2017;42:3454–65. https://doi.org/10.1016/j.ijhydene.2016.09.111.
Li Z, Chen Z, Ye H, Wang Y, Luo W, Chang J-S, Li Q, He N. Anaerobic codigestion of sewage sludge and food waste for hydrogen and VFA production with microbial community analysis. Waste Manag. 2018;78:789–99. https://doi.org/10.1016/j.wasman.2018.06.046.
Mahato RK, Kumar D, Rajagopalan G. Biohydrogen production from fruit waste by Clostridium strain BOH3. Renew Energy. 2020;153:1368–77. https://doi.org/10.1016/j.renene.2020.02.092.
Srivastava S, Kumar A, Pandey A, Pandey A. Intensification of hydrogen production by B. licheniformis using kitchen waste as substrate. Int J Hydrogen Energ. 2017;42(34):21659–66. https://doi.org/10.1016/j.ijhydene.2017.06.140.
Vu DH, Wainaina S, Taherzadeh MJ, Åkesson D, Ferreira JA. Production of polyhydroxyalkanoates (PHAs) by Bacillus megaterium using food waste acidogenic fermentation-derived volatile fatty acids. Bioengineered. 2021;12:2480–98. https://doi.org/10.1080/21655979.2021.1935524.
Farah NO, Norrsquo Aini AR, Halimatun SH, Tabassum M, Phang LY, Mohd AH. Utilization of kitchen waste for the production of green thermoplastic polyhydroxybutyrate (PHB) by Cupriavidus necator CCGUG 52238. African J Microbiol Res. 2011;5(19):2873–9. https://doi.org/10.5897/AJMR11.156.
Hafuka A, Sakaida K, Satoh H, Takahashi M, Watanabe Y, Okabe S. Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresour Technol. 2011;102(3):3551–3. https://doi.org/10.1016/j.biortech.2010.09.018.
Martino L, Cruz MV, Scoma A, Freitas F, Bertin L, Scandola M, Reis MA. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int J Biol Macromol. 2014;71:117–23. https://doi.org/10.1016/j.ijbiomac.2014.04.016.
Ji M, Zheng T, Wang Z, Lai W, Zhang L, Zhang Q, Yang H, Meng S, Xu W, Zhao C, Wu Q, Chen G-Q. PHB production from food waste hydrolysates by Halomonas bluephagenesis harboring PHB operon linked with an essential gene. Metab Eng. 2023;77:12–20. https://doi.org/10.1016/j.ymben.2023.03.003.
Borrero-de Acuña JM, Aravena-Carrasco C, Gutierrez-Urrutia I, Duchens D, Poblete-Castro I. Enhanced synthesis of medium-chain-length poly (3-hydroxyalkanoates) by inactivating the tricarboxylate transport system of Pseudomonas putida KT2440 and process development using waste vegetable oil. Process Biochem. 2019;77:23–30. https://doi.org/10.1016/j.procbio.2018.10.012.
Sarkar D, Prajapati S, Poddar K, Sarkar A. Production of ethanol by Enterobacter sp EtK3 during fruit waste biotransformation. Int Biodeterior Biodegrad. 2019;145:1–7. https://doi.org/10.1016/j.ibiod.2019.104795.
Dhiman SS, David A, Shrestha N, Johnson GR, Benjamin KM, Gadhamshetty V, Sani RK. Simultaneous hydrolysis and fermentation of unprocessed food waste into ethanol using thermophilic anaerobic bacteria. Bioresour Technol. 2017;244:733–40. https://doi.org/10.1016/j.biortech.2017.07.102.
Ma H-Z, **ng Y, Yu M, Wang Q. Feasibility of converting lactic acid to ethanol in food waste fermentation by immobilized lactate oxidase. Appl Energy. 2014;129:89–93. https://doi.org/10.1016/j.apenergy.2014.04.098.
Bibra M, Rathinam NK, Johnson GR, Sani RK. Single pot biovalorization of food waste to ethanol by Geobacillus and Thermoanaerobacter spp. Renew Energy. 2020;155:1032–41. https://doi.org/10.1016/j.renene.2020.02.093.
Ma K, Ruan Z, Shui Z, Wang Y, Hu G, He M. Open fermentative production of fuel ethanol from food waste by an acid-tolerant mutant strain of Zymomonas mobilis. Bioresour Technol. 2016;203:295–302. https://doi.org/10.1016/j.biortech.2015.12.054.
Huang H, Singh V, Qureshi N. Butanol production from food waste: a novel process for producing sustainable energy and reducing environmental pollution. Biotechnol Biofuels. 2015;8:147. https://doi.org/10.1186/s13068-015-0332-x.
Li Y, Liu Z, Ge X. Metabolic checkpoint aldehyde dehydrogenases are important for diverting β-oxidation into 1-butanol biosynthesis from kitchen waste oil in Pseudomonas aeruginosa. Appl Biochem Biotech. 2020;193:730–42. https://doi.org/10.1007/s12010-020-03456-x.
Tang J, Wang X, Hu Y, Zhang Y, Li Y. Lactic acid fermentation from food waste with indigenous microbiota: effects of pH, temperature and high OLR. Waste Manag. 2016;52:278–85. https://doi.org/10.1016/j.wasman.2016.03.034.
Yang L, Chen L, Li H, Deng Z, Liu J. Lactic acid production from mesophilic and thermophilic fermentation of food waste at different pH. J Environ Manage. 2022;304:114312. https://doi.org/10.1016/j.jenvman.2021.114312.
Peinemann JC, Demichelis F, Fiore S, Pleissner D. Techno-economic assessment of non-sterile batch and continuous production of lactic acid from food waste. Bioresour Technol. 2019;289:121631. https://doi.org/10.1016/j.biortech.2019.121631.
Mishra M, Chauhan S, Velramar B, Soni RK, Pamidimarri SDVN. Facile bioconversion of vegetable food waste into valuable organic acids and green fuels using synthetic microbial consortium. Korean J Chem Eng. 2021;38(4):833–42. https://doi.org/10.1007/s11814-020-0735-7.
Tashiro Y, Matsumoto H, Miyamoto H, Okugawa Y, Pramod P, Miyamoto H, Sakai K. A novel production process for optically pure L-lactic acid from kitchen refuse using a bacterial consortium at high temperatures. Bioresour Technol. 2013;146:672–81. https://doi.org/10.1016/j.biortech.2013.07.102.
Dessie W, Zhang W, **n F, Dong W, Zhang M, Ma J, Jiang M. Succinic acid production from fruit and vegetable wastes hydrolyzed by on-site enzyme mixtures through solid state fermentation. Bioresour Technol. 2018;247:1177–80. https://doi.org/10.1016/j.biortech.2017.08.171.
** Q, Fang Q, Chen Y, Ding W, **ao Y, Wang Z, Zhou W. Effect of Fe3O4 on propionic acid production by anaerobic fermentation of waste cooking oil and aerobic sludge. J Water Process Eng. 2022;49:102910. https://doi.org/10.1016/j.jwpe.2022.102910.
Li X, Zhang W, Ma L, Lai S, Zhao S, Chen Y, Liu Y. Improved production of propionic acid driven by hydrolyzed liquid containing high concentration of L-lactic acid from co-fermentation of food waste and sludge. Bioresour Technol. 2016;220:523–9. https://doi.org/10.1016/j.biortech.2016.08.066.
Hasan MM, Marzan LW, Honsa A, Hakim A, Azad AK. Optimisation of some fermentation conditions for the production of extracellular amylases by using Chryseobacterium and Bacillus isolates from organic kitchen wastes. J Gene Eng Biotech. 2017;6:1–8. https://doi.org/10.1016/j.jgeb.2017.02.009.
Saini V, Bhattacharya A, Gupta A. Effectiveness of sal deoiled seed cake as an inducer for protease production from Aeromonas sp. S1 for its application in kitchen wastewater treatment. Appl Biochem Biotechnol. 2013;170(8):1896–908. https://doi.org/10.1007/s12010-013-0323-y.
Dhanarajan G, Mandal M, Sen R. A combined artificial neural network modeling–particle swarm optimization strategy for improved production of marine bacterial lipopeptide from food waste. Biochem Eng J. 2014;84:59–65. https://doi.org/10.1016/j.bej.2014.01.002.
Chen C, Sun N, Li D, Long S, Tang X, **ao G, Wang L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ Sci Pollut Res. 2018;25(15):14934–43. https://doi.org/10.1007/s11356-018-1691-1.
Pan FD, Liu S, Xu QM, Chen XY, Cheng JS. Bioconversion of kitchen waste to surfactin via simultaneous enzymolysis and fermentation using mixed-culture of enzyme-producing fungi and Bacillus amyloliquefaciens HM618. Biochem Eng J. 2021;172:108036. https://doi.org/10.1016/j.bej.2021.108036.
Li P, Li T, Zeng Y, Li X, Jiang X, Wang Y, **e T, Zhang Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr Polym. 2016;151:684–91. https://doi.org/10.1016/j.carbpol.2016.06.017.
Demirci AS, Palabiyik I, Apaydın D, Mirik M, Gumus T. Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: process optimization and fermentation kinetics. LWT. 2019;101:40–7. https://doi.org/10.1016/j.lwt.2018.11.018.
Peng X-Y, Wang S-P, Chu X-L, Sun Z-Y, **a Z-Y, **e C-Y, Gou M, Tang Y-Q. Valorizing kitchen waste to produce value-added fertilizer by thermophilic semi-continuous composting followed by static stacking: performance and bacterial community succession analysis. Bioresour Technol. 2023;373:128732. https://doi.org/10.1016/j.biortech.2023.128732.
Hu J, Cai W, Wang C, Du X, Lin J, Cai J. Purification and characterization of alkaline lipase production by Pseudomonas aeruginosa HFE733 and application for biodegradation in food wastewater treatment. Biotechnol Biotechnol Equip. 2018;32:583–90. https://doi.org/10.1080/13102818.2018.1446764.
• Chong JWR, Khoo KS, Yew GY, Leong WH, Lim JW, Lam MK, et al. Advances in production of bioplastics by microalgae using food waste hydrolysate and wastewater: a review. Bioresour Technol. 2021;342:125947. https://doi.org/10.1016/j.biortech.2021.125947. This review introduced the excellent KWAKWW treatment performance of microalgae and suggested utilizing genetic engineering, transcriptional engineering, and microalgae-bacteria consortia to strengthen the treatment ability of microalgae.
Rude K, Yothers C, Barzee TJ, Kutney S, Zhang R, Franz A. Growth potential of microalgae on ammonia-rich anaerobic digester effluent for wastewater remediation. Algal Res. 2022;62: 102613. https://doi.org/10.1016/j.algal.2021.102613.
Katam K, Bhattacharyya D. Comparative study on treatment of kitchen wastewater using a mixed microalgal culture and an aerobic bacterial culture: kinetic evaluation and FAME analysis. Environ Sci Pollut Res. 2018;25:20732–742. https://doi.org/10.1007/s11356-018-2209-6.
Thanigaivel S, Vickram S, Manikandan S, Deena SR, Subbaiya R, Karmegam N, Govarthanan M, Kim W. Sustainability and carbon neutralization trends in microalgae bioenergy production from wastewater treatment: a review. Bioresour Technol. 2022;364:128057. https://doi.org/10.1016/j.biortech.2022.128057.
Rasineni GK, Loh PC, Lim BH. Characterization of chlamydomonas ribulose-1, 5-bisphosphate carboxylase/oxygenase variants mutated at residues that are posttranslationally modified. BBA-Gen Subjects. 2017;1861(Issue 2):79–85. https://doi.org/10.1016/j.bbagen.2016.10.027.
Boatman TG, Mangan NM, Lawson T, Geider RJ. Inorganic carbon and pH dependency of photosynthetic rates in Trichodesmium. J Exp Bot. 2018;69(15):3651–60. https://doi.org/10.1093/jxb/ery141.
Giordano M, Beardall J, Raven JA. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol. 2005;56:99–131. https://doi.org/10.1146/annurev.arplant.56.032604.144052.
Mukhopadhyay S, Jana A, Ghosh S, Majumdar S, Ghosh TK. Arthrospira sp. mediated bioremediation of gray water in ceramic membrane based photobioreactor: process optimization by response surface methodology. Int J Phytoremediat. 2022;24(13):1364–75. https://doi.org/10.1080/15226514.2022.2027865.
• De Bhowmick G, Sen R, Sarmah AK. Consolidated bioprocessing of wastewater cocktail in an algal biorefinery for enhanced biomass, lipid and lutein production coupled with efficient CO2 capture: An advanced optimization approach. J Environ Manage. 2019;252:109696. https://doi.org/10.1016/j.jenvman.2019.109696. This paper manifested the illustrious nutrient removal, carbon fixation, and resource utilization ability of microalgae.
Kumar PK, Krishna SV, Naidu SS, Verma K, Bhagawan D, Himabindu V. Biomass production from microalgae Chlorella grown in sewage, kitchen wastewater using industrial CO2 emissions: comparative study. Carbon Resour Convers. 2019;2:126–33. https://doi.org/10.1016/j.crcon.2019.06.002.
Tian C, Ye X, Xu Y, Hua W, Wang W, Wu S, et al. Enhanced microalgae cultivation using digested kitchen waste sewage treated with struvite precipitation. Int J Agric Biol Eng. 2017;10(1):142–47. https://doi.org/10.3965/j.ijabe.20171001.2318.
Kamyab H, Chelliapan S, Lee CT, Khademi T, Kumar A, Yadav KK, Rezania S, Kumar S, Ebrahimi SS. Improved production of lipid contents by cultivating Chlorella pyrenoidosa in heterogeneous organic substrates. Clean Technol Environ Policy. 2019;21:1969–78. https://doi.org/10.1007/s10098-019-01743-8.
Pei H, Jiang L, Hou Q, Yu Z. Toward facilitating microalgae cope with effluent from anaerobic digestion of kitchen waste: the art of agricultural phytohormones. Biotechnol Biofuels. 2017;10:1–18. https://doi.org/10.1186/s13068-017-0759-3.
Tan XB, Wang L, Wan XP, Zhou XN, Yang LB, Zhang WW, Zhao XC. Growth of Chlorella pyrenoidosa on different septic tank effluents from rural areas for lipids production and pollutants removal. Bioresour Technol. 2021;339:125502. https://doi.org/10.1016/j.biortech.2021.125502.
Nwoba EG, Mickan BS, Moheimani NR. Chlorella sp. growth under batch and fed-batch conditions with effluent recycling when treating the effluent of food waste anaerobic digestate. J Appl Phycol. 2019;31:3545–56. https://doi.org/10.1007/s10811-019-01878-7.
Shin DY, Cho HU, Utomo JC, Choi YN, Xu X, Park JM. Biodiesel production from Scenedesmus bijuga grown in anaerobically digested food wastewater effluent. Bioresour Technol. 2015;184:215–21. https://doi.org/10.1016/j.biortech.2014.10.090.
Babu A, Katam K, Gundupalli MP, Bhattacharyya D. Nutrient removal from wastewater using microalgae: a kinetic evaluation and lipid analysis. Water Environ Res. 2018;90(6):520–9. https://doi.org/10.2175/106143017X15054988926299.
Castillo T, Ramos D, García-Beltrán T, Brito-Bazan M, Galindo E. Mixotrophic cultivation of microalgae: an alternative to produce high-value metabolites. Biochem Eng J. 2021;176:108183. https://doi.org/10.1016/j.bej.2021.108183.
Pradhan N, Kumar S, Selvasembian R, Rawat S, Gangwar A, Senthamizh R, Yuen YK, Luo L, Ayothiraman S, Saratale GD, Mal J. Emerging trends in the pretreatment of microalgal biomass and recovery of value-added products: a review. Bioresour Technol. 2023;369:128395. https://doi.org/10.1016/j.biortech.2022.128395.
Rastogi RP, Sonani RR, Madamwar D, Incharoensakdi A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016;16:110–8. https://doi.org/10.1016/j.algal.2016.03.009.
Wang X, Zhang MM, Liu SF, Xu RL, Mou JH, Qin ZH, Zhou ZG, Li HY, Lin CSK, Sun Z. Synergistic bioconversion of lipids and carotenoids from food waste by Dunaliella salina with fulvic acid via a two-stage cultivation strategy. Energy Convers Manag. 2021;234:113908. https://doi.org/10.1016/j.enconman.2021.113908.
Zhai Q, Hong Y, Wang X, Wang Q, Zhao G, Liu X, et al. Mixing starch wastewaters to balance nutrients for improving nutrient removal, microalgae growth and accumulation of high value-added products. Water Cycle. 2022;3:151–59. https://doi.org/10.1016/j.watcyc.2022.09.004.
Sharma S, Show PL, Aminabhavi TM, Sevda S, Garlapati VK. Valorization of environmental-burden waste towards microalgal metabolites production. Environ Res. 2023;227:115320. https://doi.org/10.1016/j.envres.2023.115320.
Amorim ML, Soares J, Coimbra JSDR. Leite MdO, Albino LFT, Martins MA. Microalgae proteins: production, separation, isolation, quantification, and application in food and feed. Crit Rev Food Sci Nutr. 2021;61(12):1976–2002. https://doi.org/10.1080/10408398.2020.1768046.
Sui Y, Vlaeminck SE. Dunaliella microalgae for nutritional protein: an undervalued asset. Trends Biotechnol. 2020;38:10–2. https://doi.org/10.1016/j.tibtech.2019.07.011.
Sui Y, Muys M, Vermeir P, D’Adamo S, Vlaeminck SE. Light regime and growth phase affect the microalgal production of protein quantity and quality with Dunaliella salina. Bioresour Technol. 2019;275:145–52. https://doi.org/10.1016/j.biortech.2018.12.046.
Zhang L, Cheng J, Pei H, Pan J, Jiang L, Hou Q, Han F. Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew Energy. 2018;115:276–87. https://doi.org/10.1016/j.renene.2017.08.034.
Khoo KS, Ahmad I, Chew KW, Iwamoto K, Bhatnagar A, Show PL. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: a review. Prog Energy Combust Sci. 2023;96:101071. https://doi.org/10.1016/j.pecs.2023.101071.
Sajjadi B, Chen W-Y, Raman AAA, Ibrahim S. Microalgae lipid and biomass for biofuel production: a comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew Sustain Energy Rev. 2018;97:200–32. https://doi.org/10.1016/j.rser.2018.07.050.
Tang DYY, Yew GY, Koyande AK, Chew KW, Vo DVN, Show PL. Green technology for the industrial production of biofuels and bioproducts from microalgae: a review. Environ Chem Lett. 2020;1:19. https://doi.org/10.1007/s10311-020-01052-3.
Sharma KK, Schuhmann H, Schenk PM. High lipid induction in microalgae for biodiesel production. Energies. 2012;5:1532–53. https://doi.org/10.3390/en5051532.
Maltsev Y, Maltseva K. Fatty acids of microalgae: diversity and applications. Rev Environ Sci Biotechnol. 2021;20(2):515–47. https://doi.org/10.1007/s11157-021-09571-3.
Karpagam R, Abinaya N, Gnanam R. Assortment of native microalgae for improved biomass and lipid production on employing vegetable waste as a frugal cultivation approach for biodiesel application. Curr Microbiol. 2021;78(10):3770–81. https://doi.org/10.1007/s00284-021-02643-1.
Feng PZ, Xu ZB, Qin L, Alam MA, Wang ZM, Zhu SN. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour Technol. 2020;301:122726. https://doi.org/10.1016/j.biortech.2020.122762.
Liu Z, Gao Y, Chen J, Imanaka T, Bao J, Hua Q. Analysis of metabolic fluxes for better understanding of mechanisms related to lipid accumulation in oleaginous yeast Trichosporon cutaneum. Bioresour Technol. 2013;130:144–51. https://doi.org/10.1016/j.biortech.2012.12.072.
Choi YY, Patel AK, Hong ME, Chang WS, Sim SJ. Microalgae Bioenergy with Carbon Capture and Storage (BECCS): an emerging sustainable bioprocess for reduced CO2 emission and biofuel production. Bioresour Technol Rep. 2019;7:100270. https://doi.org/10.1016/j.biteb.2019.100270.
De Bhowmick G, Sarmah AK, Sen R. Performance evaluation of an outdoor algal biorefinery for sustainable production of biomass, lipid and lutein valorizing flue-gas carbon dioxide and wastewater cocktail. Bioresour Technol. 2019;283:198–206. https://doi.org/10.1016/j.biortech.2019.03.075.
Liang GB, Mo YW, Zhou QF. Optimization of digested chicken manure filtrate supplementation for lipid overproduction in heterotrophic culture Chlorella protothecoides. Fuel. 2013;108:159–65. https://doi.org/10.1016/j.fuel.2013.02.003.
Lin TS, Wu JY. Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour Technol. 2015;184:100–7. https://doi.org/10.1016/j.biortech.2014.11.005.
Bibi F, Jamal A, Huang Z, Urynowicz M, Ali MI. Advancement and role of abiotic stresses in microalgae biorefinery with a focus on lipid production. Fuel. 2022;316:123192. https://doi.org/10.1016/j.fuel.2022.123192.
• Pei H, Zhang L, Betenbaugh MJ, Jiang L, Lin X, Ma C, et al. Highly efficient harvesting and lipid extraction of limnetic Chlorella sorokiniana SDEC-18 grown in seawater for microalgal biofuel production. Algal Res. 2022;66:102813. https://doi.org/10.1016/j.algal.2022.102813. This article emphasized that using seawater to cultivate microalgae could improve the lipids productivity of microalgae and the extraction efficiency of microalgal lipids.
de Medeiros VPB, Pimentel TC, Varandas RCR, Dos Santos SA, de Souza Pedrosa GT, da Costa Sassi CF, da Conceição MM, Magnani M. Exploiting the use of agro-industrial residues from fruit and vegetables as alternative microalgae culture medium. Food Res Int. 2020;137:109722. https://doi.org/10.1016/j.foodres.2020.109722.
Koçer AT, İnan B, Özçimen D, Gökalp İ. A study of microalgae cultivation in hydrothermal carbonization process water: nutrient recycling, characterization and process design. Environ Technol Innov. 2023;30: 103048. https://doi.org/10.1016/j.eti.2023.103048.
Cheng J, Ye Q, Xu J, Yang ZB, Zhou JH, Cen KF. Improving pollutants removal by microalgae Chlorella PY-ZU1 with 15% CO2 from undiluted anaerobic digestion effluent of food wastes with ozonation pretreatment. Bioresour Technol. 2016;216:273–9. https://doi.org/10.1016/j.biortech.2016.05.069.
Sloth JK, Jensen HC, Pleissner D, Eriksen NT. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour Technol. 2017;238:296–305. https://doi.org/10.1016/j.biortech.2017.04.043.
Haske-Cornelius O, Vu T, Schmiedhofer C, Vielnascher R, Dielacher M, Sachs V, Grasmug M, Kromus S, Guebitz GM. Cultivation of heterotrophic algae on enzymatically hydrolyzed municipal food waste. Algal Res. 2020;50:101993. https://doi.org/10.1016/j.algal.2020.101993.
Chi Z, Zheng Y, Jiang A, Chen S. Lipid production by culturing oleaginous yeast and algae with food waste and municipal wastewater in an integrated process. Appl Biochem Biotechnol. 2011;165:442–53. https://doi.org/10.1007/s12010-011-9263-6.
Pleissner D, Lam WC, Sun Z, Lin CSK. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour Technol. 2013;137:139–46. https://doi.org/10.1016/j.biortech.2013.03.088.
Lau KY, Pleissner D, Lin CSK. Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresour Technol. 2014;170:144–51. https://doi.org/10.1016/j.biortech.2014.07.096.
Pleissner D, Lau KY, Ki Lin CS. Utilization of food waste in continuous flow cultures of the heterotrophic microalga Chlorella pyrenoidosa for saturated and unsaturated fatty acids production. J Clean Prod. 2017;142(Part 4):1417–24. https://doi.org/10.1016/j.jclepro.2016.11.165.
Yu Z, Song M, Pei H, Han F, Jiang L, Hou Q. The growth characteristics and biodiesel production of ten algae strains cultivated in anaerobically digested effluent from kitchen waste. Algal Res. 2017;24:265–75. https://doi.org/10.1016/j.algal.2017.04.010.
Wang X, Liu SF, Qin ZH, Balamurugan S, Li HY, Lin CSK. Sustainable and stepwise waste-based utilisation strategy for the production of biomass and biofuels by engineered microalgae. Environ Pollut. 2020;265:114854. https://doi.org/10.1016/j.envpol.2020.114854.
Almutairi AW, Al-Hasawi ZM, Abomohra AEF. Valorization of lipidic food waste for enhanced biodiesel recovery through two-step conversion: a novel microalgae-integrated approach. Bioresour Technol. 2021;342: 125966. https://doi.org/10.1016/j.biortech.2021.125966.
Wang X, Liu SF, Wang ZY, Hao TB, Balamurugan S, Li DW, et al. A waste upcycling loop: Two-factor adaptive evolution of microalgae to increase polyunsaturated fatty acid production using food waste. J Clean Prod. 2022;331:130018. https://doi.org/10.1016/j.jclepro.2021.130018.
Wang X, Balamurugan S, Liu S-F, Zhang M-M, Yang W-D, Liu J-S, Li H-Y, Lin CSK. Enhanced polyunsaturated fatty acid production using food wastes and biofuels byproducts by an evolved strain of Phaeodactylum tricornutum. Bioresour Technol. 2020;296:122351. https://doi.org/10.1016/j.biortech.2019.122351.
Patel A, Hruzova K, Rova U, Christakopoulos P, Matsakas L. Sustainable biorefinery concept for biofuel production through holistic volarization of food waste. Bioresour Technol. 2019;294:122247. https://doi.org/10.1016/j.biortech.2019.122247.
Tale M, Ghosh S, Kapadnis B, Kale S. Isolation and characterization of microalgae for biodiesel production from Nisargruna biogas plant effluent. Bioresour Technol. 2014;169:328–35. https://doi.org/10.1016/j.biortech.2014.06.017.
Wang X, Zhang MM, Sun Z, Liu SF, Qin ZH, Mou JH, Zhou ZG, Lin CSK. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J Hazard Mater. 2020;400:123258. https://doi.org/10.1016/j.jhazmat.2020.123258.
Liu M, Yu Z, Jiang L, Hou Q, **e Z, Ma M, Yu S, Pei H. Monosodium glutamate wastewater assisted seawater to increase lipid productivity in single-celled algae. Renew Energ. 2021;179:1793–802. https://doi.org/10.1016/j.renene.2021.08.006.
Jiang L, Zhang L, Nie C, Pei H. Lipid productivity in limnetic Chlorella is doubled by seawater added with anaerobically digested effluent from kitchen waste. Biotechnol Biofuels. 2018;11:68. https://doi.org/10.1186/s13068-018-1064-5.
De Bhowmick G, Sen R, Sarmah AK. Analysis of growth and intracellular product synthesis dynamics of a microalga cultivated in wastewater cocktail as medium. Biochem Eng J. 2019;149:107253. https://doi.org/10.1016/j.bej.2019.107253.
• Talapatra N, Ghosh UK. New concept of biodiesel production using food waste digestate powder: Co-culturing algae-activated sludge symbiotic system in low N and P paper mill wastewater. Sci Total Environ. 2022;844:157207. https://doi.org/10.1016/j.scitotenv.2022.157207. This article indicated that microalgae-bacteria consortia could efficiently remove nutrients and accumulate large amounts of lipids from ADE-KW.
Sutherland DL, Burke J, Leal E, Ralph PJ. Effects of nutrient load on microalgal productivity and community composition grown in anaerobically digested food-waste centrate. Algal Res. 2020;51:102037. https://doi.org/10.1016/j.algal.2020.102037.
Sutherland DL, Bramucci A. Dissolved organic phosphorus bioremediation from food-waste centrate using microalgae. J Environ Manag. 2022;313:115018. https://doi.org/10.1016/j.jenvman.2022.115018.
• Li B, Bao M, Liu Y, Cheng L, Cui B, Hu Z. Novel shortcut biological nitrogen removal using activated sludge-biofilm coupled with symbiotic algae. J Water Process Eng. 2021;43:102275. https://doi.org/10.1016/j.jhazmat.2020.123258. This article found that microalgae-bacteria consortia enhanced their nutrient purification ability and reduced operation costs through secreting HA and releasing O2.
Lipczynska-Kochany E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: a review. Chemosphere. 2018;202:420–37. https://doi.org/10.1016/j.chemosphere.2018.03.104.
Zhang S, Su J, Ali A, Zheng Z, Sun Y. Enhanced denitrification performance of strain YSF15 by different molecular weight of humic acid: Mechanism based on the biological products and activity. Bioresour Technol. 2021;325:124709. https://doi.org/10.1016/j.biortech.2021.124709.
Albers CN, Ellegaard-Jensen L, Hansen LH, Sorensen SR. Bioaugmentation of rapid sand filters by microbiome priming with a nitrifying consortium will optimize production of drinking water from groundwater. Water Res. 2018;129:1–10. https://doi.org/10.1016/j.watres.2017.11.009.
Marín D, Méndez L, Suero I, Díaz I, Blanco S, Fdz-Polanco M, et al. Anaerobic digestion of food waste coupled with biogas upgrading in an outdoors algal-bacterial photobioreactor at pilot scale. Fuel. 2022;324(Part A):124554. https://doi.org/10.1016/j.fuel.2022.124554.
Chen X, Hu Z, Qi Y, Song C, Chen G. The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Bioresour Technol. 2019;292:122017. https://doi.org/10.1016/j.biortech.2019.122017.
Fernandes F, Silkina A, Gayo-Peláez JI, Kapoore RV, de la Broise D, Llewellyn CA. Microalgae cultivation on nutrient rich digestate: the importance of strain and digestate tailoring under PH control. Appl Sci. 2022;12:5429. https://doi.org/10.3390/app12115429.
Leong WH, Lim JW, Lam MK, Uemura Y, Ho CD, Ho YC. Co-cultivation of activated sludge and microalgae for the simultaneous enhancements of nitrogen-rich wastewater bioremediation and lipid production. J Taiwan Inst Chem Eng. 2018;87:216–24. https://doi.org/10.1016/j.jtice.2018.03.038.
Zhang C, Li S, Ho S-H. Converting nitrogen and phosphorus wastewater into bioenergy using microalgae-bacteria consortia: a critical review. Bioresour Technol. 2021;342:126056. https://doi.org/10.1016/j.biortech.2021.126056.
Manhaeghe D, Allosserie A, Rousseau DPL, Van Hulle SWH. Model based analysis of carbon fluxes within microalgae-bacteria flocs using respirometric-titrimetric data. Sci Total Environ. 2021;784:147048. https://doi.org/10.1016/j.scitotenv.2021.147048.
Fallahi A, Rezvani F, Asgharnejad H, Nazloo EK, Ha**ajaf N, Higgins B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: a review. Chemosphere. 2021;272:129878. https://doi.org/10.1016/j.chemosphere.2021.129878.
Li L, Liu W, Liang T, Ma F. The adsorption mechanisms of algae-bacteria symbiotic system and its fast formation process. Bioresour Technol. 2020;315:123854. https://doi.org/10.1016/j.biortech.2020.123854.
Matho C, Schwarzenberger K, Eckert K, Keshavarzi B, Walther T, Steingroewer J, Krujatz F. Bio-compatible flotation of Chlorella vulgaris: study of zeta potential and flotation efficiency. Algal Res. 2019;44:101705. https://doi.org/10.1016/j.algal.2019.101705.
Wang X, Dong H-P, Wei W, Balamurugan S, Yang W-D, Liu J-S, Li H-Y. Dual expression of plastidial GPAT1 and LPAT1 regulates triacylglycerol production and the fatty acid profile in Phaeodactylum tricornutum. Biotechnol Biofuels. 2018;11:318. https://doi.org/10.1186/s13068-018-1317-3.
Rathod JP, Vira C, Lali AM, Prakash G. Metabolic engineering of Chlamydomonas reinhardtii for enhanced beta-carotene and lutein production. Appl Biochem Biotechnol. 2020;190(4):1457–69. https://doi.org/10.1007/s12010-019-03194-9.
Fang H, Li D, Kang J, Jiang P, Sun J, Zhang D. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat Commun. 2018;9:4917. https://doi.org/10.1038/s41467-018-07412-6.
Jiang L, Li Y, Pei H. Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew Sustain Energy Rev. 2021;149:111395. https://doi.org/10.1016/j.rser.2021.111395.
Wu H, Yang J, Shen P, Li Q, Wu W, Jiang X, Qin L, Huang J, Cao X, Qi F. High-level production of indole-3-acetic acid in the metabolically engineered Escherichia coli. J Agric Food Chem. 2021;69:1916–24. https://doi.org/10.1021/acs.jafc.0c08141.
Kumar SD, Yasasve M, Karthigadevi G, Aashabharathi M, Subbaiya R, Karmegam N, Govarthanan M. Efficiency of microbial fuel cells in the treatment and energy recovery from food wastes: trends and applications-a review. Chemosphere. 2022;287:132439. https://doi.org/10.1016/j.chemosphere.2021.132439.
Hou Q, Yang Z, Chen S, Pei H. Using an anaerobic digestion tank as the anodic chamber of an algae-assisted microbial fuel cell to improve energy production from food waste. Water Res. 2020;170:115305. https://doi.org/10.1016/j.watres.2019.115305.
Yoshizu D, Kouzuma A, Watanabe K. Use of microbial fuel cells for the treatment of residue effluents discharged from an anaerobic digester treating food wastes. Microorganisms. 2023;11(3):598. https://doi.org/10.3390/microorganisms11030598.
Chen D, Shen J, Jiang X, Su G, Han W, Sun X, Li J, Mu Y, Wang L. Simultaneous debromination and mineralization of bromophenol in an up-flow electricity-stimulated anaerobic system. Water Res. 2019;157:8–18. https://doi.org/10.1016/j.watres.2019.03.054.
Hussain A, Lee J, **ong Z, Wang Y, Lee H-S. Butyrate production and purification by combining dry fermentation of food waste with a microbial fuel cell. J Environ Manage. 2021;300:113827. https://doi.org/10.1016/j.jenvman.2021.113827.
Lafi H, Panu U, Liao B. Effect of the organic carbon to nutrient (N and P) ratio on the biological performance of a microalgal–bacterial membrane photobioreactor. Environ Sci: Water Res Technol. 2023;9:2021–30. https://doi.org/10.1039/D3EW00117B.
Segredo-Morales E, González E, González-Martín C, Vera L. Secondary wastewater effluent treatment by microalgal-bacterial membrane photobioreactor at long solid retention times. J Water Process Eng. 2022;49:103200. https://doi.org/10.1016/j.jwpe.2022.103200.
Aydin S, Ünlü İD, Arabacı DN, Duru ÖA. Evaluating the effect of microalga Haematococcus pluvialis bioaugmentation on aerobic membrane bioreactor in terms of performance, membrane fouling and microbial community structure. Sci Total Environ. 2022;807:149908. https://doi.org/10.1016/j.scitotenv.2021.149908.
Yu Z, Pei H, Li Y, Yang Z, **e Z, Hou Q, Nie C. Inclined algal biofilm photobioreactor (IABPBR) for cost-effective cultivation of lipid-rich microalgae and treatment of seawater-diluted anaerobically digested effluent from kitchen waste with the aid of phytohormones. Bioresour Technol. 2020;315:123761. https://doi.org/10.1016/j.biortech.2020.123761.
Ritigala T, Chen Y, Zheng J, Demissie H, Zheng L, Yu D, Sui Q, Chen M, Zhu J, Fan H, Li J, Gao Q, Weragoda SK, Weerasooriya R, **adasa K, Wei Y. Comparison of an integrated short-cut biological nitrogen removal process with magnetic coagulation treating swine wastewater and food waste digestate. Bioresour Technol. 2021;329:124904. https://doi.org/10.1016/j.biortech.2021.124904.
Yin J, Jiang J, Tang Q. Advanced treatment of digested restaurant wastewater using a combination of anaerobic/oxicunit, Fenton, and a biological aerated filter in pilot-scale treatment. Water Air Soil Poll. 2023;234:92. https://doi.org/10.1007/s11270-023-06106-0.
Yang Z, Nie C, Hou Q, Zhang L, Zhang S, Yu Z, Pei H. Coupling a photosynthetic microbial fuel cell (PMFC) with photobioreactors (PBRs) for pollutant removal and bioenergy recovery from anaerobically digested effluent. Chem Eng J. 2019;359:402–8. https://doi.org/10.1016/j.cej.2018.11.136.
Tahmasebi P, Kamrava S, Bai T, Sahimi M. Machine learning in geo- and environmental sciences: from small to large scale. Adv Water Resour. 2020;142:103619. https://doi.org/10.1016/j.advwatres.2020.103619.
Gurjar R, Behera M. Optimization and modelling of volatile fatty acid generation in a leachate bed reactor for utilization in microbial fuel cells. Water Environ J. 2023;37(3):581–93. https://doi.org/10.1111/wej.12861.
Long F, Fan J, Liu H. Prediction and optimization of medium-chain carboxylic acids production from food waste using machine learning models. Bioresour Technol. 2023;370:128533. https://doi.org/10.1016/j.biortech.2022.128533.
Long F, Fan J, Xu W, Liu H. Predicting the performance of medium-chain carboxylic acid (MCCA) production using machine learning algorithms and microbial community data. J Clean Prod. 2022;377:134223. https://doi.org/10.1016/j.jclepro.2022.134223.
Funding
This research is funded by the National Natural Science Foundation of China (No. 52270021) and the Scientific Research Project supported by Enterprise (No.2023-HXFW-0040, No.2023-HXFW-0025, and No.2022-HXFW-0002).
Author information
Authors and Affiliations
Contributions
Z.W. wrote the manuscript and prepared all the tables and figures. Y.H. provided funding and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Human and Animal Rights and Informed Consent
This article does not contain any study with human and animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Hong, Y. Microbial-Based Treatment of Kitchen Waste and Kitchen Wastewater: State-of-the-Art Progress and Emerging Research Prospects Related to Microalgae and Bacteria. Curr Pollution Rep 10, 139–171 (2024). https://doi.org/10.1007/s40726-024-00300-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-024-00300-2