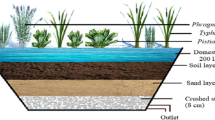
Abstract
Purpose of Review
Constructed wetlands (CWs) are engineered systems that have been proven as an alternative option to traditional wastewater treatment technologies because of their ability to provide cost-effective and energy-efficient solutions. This technology depends on natural microbial/biological, physical, and chemical processes to treat wastewater. Processes removing impurities in constructed wetlands are based on the combination of interactive systems such as selected plant species, the nature of substrate used for constructed wetlands, biofilm growth, microbial diversity, and several biogeochemically affected reaction cycles in wetland systems. Microorganisms play a vital role in these processes such as the degradation of pollutants and the transformation of nutrients. Microorganisms remove the pollutants from CWs by catalyzing chemical reactions, biodegrading, biosorbing, and supporting plant growth. An in-depth analysis of the function of microorganisms in CWs is important to understand. This review deals with the recent developments in constructed wetland systems from a microbiological perspective to treat impurities present in wastewater. It focuses on the studies of microbial diversity in CWs and the role of enzymes produced by microbes, the influence of the substrates of CWs on microbial diversity, the influence of the hydraulic design of CWs on the growth of microorganisms, the role of specific microbes in the removal of pollutants and the different software, analytical equipment, tools, and techniques used to measure/quantify the parameters of interest or to design and operate a wetland.
Recent Findings
The combination of different types of substrates in constructed wetlands can form different types of zones such as anaerobic and aerobic zones which can allow to form a diversity of microorganisms. In addition, plant diversity plays a vital role in microbial growth by providing O2 and increasing plant biomass production which influences the soil microbial community. Moreover, the influent carbon source influences the biomass as for example when the COD/N ratio is increased by 80%, the phospholipid fatty acids (PLFA) concentration of microbial biofilm in glucose constructed wetlands is increased by 50%. At the same time, the biomass of aerobic and anaerobic bacteria and fungi increased significantly. In addition, different microorganisms are responsible in removing different types of heavy metals and micropollutants.
Summary
This article provides useful information on the understanding of the diversity of microbes, influencing factors on the growth of microorganisms, and the efficiency of pollutant removal process in CWs. Overall, this review provides new ideas and directions for the improvement of constructed wetlands from a microbiological perspective.


Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available in the references cited in the paper.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pelissari C, Guivernau M, Viñas M, García J, Velasco-Galilea M, Souza SS, Sezerino PH, Ávila C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018;141:185–95.
Fu G, Wu J, Han J, Zhao L, Chan G, Leong K. Effects of substrate type on denitrification efficiency and microbial community structure in constructed wetlands. Biores Technol. 2020;307:123222.
Fang J, Dong J, Li C, Chen H, Wang L, Lyu T, He H, Liu J. Response of microbial community composition and function to emergent plant rhizosphere of a constructed wetland in northern China. Appl Soil Ecol. 2021;168:104141.
Adrados B, Sánchez O, Arias CA, Becares E, Garrido L, Mas J, Brix H, Morató J. Microbial communities from different types of natural wastewater treatment systems: vertical and horizontal flow constructed wetlands and biofilters. Water Res. 2014;55:304–12.
Sánchez O. Constructed wetlands revisited: microbial diversity in the–omics era. Microb Ecol. 2017;73(3):722–33.
Gagnon V, Chazarenc F, Comeau Y, Brisson J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci Technol. 2007;56(3):249–54.
Lin Y-F, **g S-R, Lee D-Y, Chang Y-F, Shih K-C. Nitrate removal from groundwater using constructed wetlands under various hydraulic loading rates. Biores Technol. 2008;99(16):7504–13.
Shahid MJ, AL-surhanee AA, Kouadri F, Ali S, Nawaz N, Afzal M, Rizwan M, Ali B,Soliman MH. Role of microorganisms in the remediation of wastewater in floating treatment wetlands: a review. Sustainability. 2020;12(14):5559.
Ge H, Batstone DJ, Keller J. Biological phosphorus removal from abattoir wastewater at very short sludge ages mediated by novel PAO clade Comamonadaceae. Water Res. 2015;69:173–82.
Lloyd J, Klessa D, Parry DL, Buck P, Brown N. Stimulation of microbial sulphate reduction in a constructed wetland: microbiological and geochemical analysis. Water Res. 2004;38(7):1822–30.
Desta AF, Assefa F, Leta S, Stomeo F, Wamalwa M, Njahira M, Appolinaire D. Microbial community structure and diversity in an integrated system of anaerobic-aerobic reactors and a constructed wetland for the treatment of tannery wastewater in Modjo, Ethiopia. PLoS ONE. 2014;9(12):e115576.
Yun J, Deng Y, Zhang H. Anthropogenic protection alters the microbiome in intertidal mangrove wetlands in Hainan Island. Appl Microbiol Biotechnol. 2017;101(15):6241–52.
Ajwang’Ondiek R, Kitaka N, Oduor SO. Assessment of provisioning and cultural ecosystem services in natural wetlands and rice fields in Kano floodplain, Kenya. Ecosyst Serv. 2016;21:166–173.
Friess DA, Yando ES, Alemu JB, Wong L-W, Soto SD, Bhatia N. Ecosystem services and disservices of mangrove forests and salt marshes. Oceanog Mar Biol. 2020.
Veettil BK, Pereira SFR, Quang NX. Rapidly diminishing mangrove forests in Myanmar (Burma): a review. Hydrobiologia. 2018;822:19–35.
Rajan RJ, Sudarsan J, Nithiyanantham S. Microbial population dynamics in constructed wetlands: review of recent advancements for wastewater treatment. Environ Eng Res. 2019;24(2):181–90.
Mina IA-P, Costa M, Matos A, Calheiros CSC,Castro P. Polishing domestic wastewater on a subsurface flow constructed wetland: organic matter removal and microbial monitoring. Int J Phytoremediation. 2011;13(10):947–958.
Hu S, Zuo X, Lv Z, He J, Wu Y, Liu H, Chen Z. Drained water quality in sludge treatment wetlands: effects of earthworm densities and plant species. J Clean Prod. 2020;247:119128.
•• Cheng R, Zhu H, Shutes B,Yan B. Treatment of microcystin (MC-LR) and nutrients in eutrophic water by constructed wetlands: performance and microbial community. Chemosphere. 2021;263:128139. This paper describes that microenvironment of ceramsite substrate based CWs is beneficial for the growth of functional microorganisms.
Du L, Trinh X, Chen Q, Wang C, Wang H, **a X, Zhou Q, Xu D, Wu Z. Enhancement of microbial nitrogen removal pathway by vegetation in integrated vertical-flow constructed wetlands (IVCWs) for treating reclaimed water. Biores Technol. 2018;249:644–51.
Nie SA, Li H, Yang X, Zhang Z, Weng B, Huang F, Zhu GB, Zhu YG. Nitrogen loss by anaerobic oxidation of ammonium in rice rhizosphere. ISME J. 2015;9(9):2059–2067.
Kadlec R. The effects of wetland vegetation and morphology on nitrogen processing. Ecol Eng. 2008;33(2):126–41.
Wang W, Zhao Y, Jiang G, Wang Y. The nutrient removal ability and microbial communities in a pilot-scale horizontal subsurface flow constructed wetland fed by slightly polluted Lake water. Wetlands. 2020;40:2085–96.
Saleem H, Arslan M, Rehman K, Tahseen R, Afzal M. Phragmites australis—a helophytic grass—can establish successful partnership with phenol-degrading bacteria in a floating treatment wetland. Saudi J Biol Sci. 2019;26(6):1179–86.
Fu G, Zhao L, Huangshen L,Wu J. Isolation and identification of a salt-tolerant aerobic denitrifying bacterial strain and its application to saline wastewater treatment in constructed wetlands. Bioresour Technol. 2019;290:121725. https://doi.org/10.1016/j.biortech.2019.121725.
Li X, Zhang M, Liu F, Chen L, Li Y, Li Y, **ao R, Wu J. Seasonality distribution of the abundance and activity of nitrification and denitrification microorganisms in sediments of surface flow constructed wetlands planted with Myriophyllum elatinoides during swine wastewater treatment. Biores Technol. 2018;248:89–97. https://doi.org/10.1016/j.biortech.2017.06.102.
Wang Q, Cao Z, Liu Q, Zhang J, Hu Y, Zhang J, Xu W, Kong Q, Yuan X, Chen Q. Enhancement of COD removal in constructed wetlands treating saline wastewater: intertidal wetland sediment as a novel inoculation. J Environ Manage. 2019;249:109398. https://doi.org/10.1016/j.jenvman.2019.109398.
Bahr M, Crump BC, Klepac-Ceraj V, Teske A, Sogin ML, Hobbie JE. Molecular characterization of sulfate-reducing bacteria in a New England salt marsh. Environ Microbiol. 2005;7(8):1175–85.
Ibekwe AM, Grieve CM, Lyon SR. Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl Environ Microbiol. 2003;69(9):5060–9.
Karajić M, Lapanje A, Razinger J, Zrimec A, Vrhovšek D. The effect of the application of halotolerant microorganisms on the efficiency of a pilot-scale constructed wetland for saline wastewater treatment. J Serb Chem Soc. 2010;75(1):129–42.
Shen Y, Zhuang L, Zhang J, Fan J, Yang T, Sun S. A study of ferric-carbon micro-electrolysis process to enhance nitrogen and phosphorus removal efficiency in subsurface flow constructed wetlands. Chem Eng J. 2019;359:706–12.
Cao W, Zhang Y. Removal of nitrogen (N) from hypereutrophic waters by ecological floating beds (EFBs) with various substrates. Ecol Eng. 2014;62:148–52.
Calheiros CS, Duque AF, Moura A, Henriques IS, Correia A, Rangel AO, Castro PM. Substrate effect on bacterial communities from constructed wetlands planted with Typha latifolia treating industrial wastewater. Ecol Eng. 2009;35(5):744–53.
Guan W, Yin M, He T, **e S. Influence of substrate type on microbial community structure in vertical-flow constructed wetland treating polluted river water. Environ Sci Pollut Res. 2015;22:16202–9.
Li M, Zhou Q, Tao M, Wang Y, Jiang L, Wu Z. Comparative study of microbial community structure in different filter media of constructed wetland. J Environ Sci. 2010;22(1):127–33.
Vacca G, Wand H, Nikolausz M, Kuschk P, Kästner M. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005;39(7):1361–73.
Stefanakis AI, Tsihrintzis VA. Effects of loading, resting period, temperature, porous media, vegetation and aeration on performance of pilot-scale vertical flow constructed wetlands. Chem Eng J. 2012;181:416–30.
Vymazal J. Plants used in constructed wetlands with horizontal subsurface flow: a review. Hydrobiologia. 2011;674(1):133–56.
Yang X, He Q, Guo F, Sun X, Zhang J, Chen Y. Impacts of carbon-based nanomaterials on nutrient removal in constructed wetlands: microbial community structure, enzyme activities, and metabolism process. J Hazard Mater. 2021;401:123270.
•• Ding Y, Lyu T, Bai S, Li Z, Ding H, You S, **e Q. Effect of multilayer substrate configuration in horizontal subsurface flow constructed wetlands: assessment of treatment performance, biofilm development, and solids accumulation. Environ Sci Pollut Res. 2018;25:1883–1891. This paper highlights that multilayer substrate configuration significantly influenced biofilm growth and solids accumulation in constructed wetlands.
Caselles-Osorio A, Puigagut J, Segú E, Vaello N, Granés F, García D, García J. Solids accumulation in six full-scale subsurface flow constructed wetlands. Water Res. 2007;41(6):1388–98.
Sanchez-Huerta C, Fortunato L, Leiknes T, Hong P-Y. Influence of biofilm thickness on the removal of thirteen different organic micropollutants via a membrane aerated biofilm reactor (MABR). J Hazard Mater. 2022;432:128698.
Han B, Zhang S, Wang P, Wang C. Effects of water flow on submerged macrophyte-biofilm systems in constructed wetlands. Sci Rep. 2018;8(1):1–12.
Morató J, Codony F, Sánchez O, Pérez LM, García J, Mas J. Key design factors affecting microbial community composition and pathogenic organism removal in horizontal subsurface flow constructed wetlands. Sci Total Environ. 2014;481:81–9.
Samsó R, García J. Bacteria distribution and dynamics in constructed wetlands based on modelling results. Sci Total Environ. 2013;461:430–40.
•• Huang L, Wang N, Deng C, Liang Y, Wang Q, Liu M, Chen Y. Interactive effect of carbon source with influent COD/N on nitrogen removal and microbial community structure in subsurface flow constructed wetlands. J Environ Manage. 2019;250:109491. This paper spotlights that microbial community structure is affected significantly by carbon source, influent COD/N ratio and their interaction.
Ye F, Li Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol Eng. 2009;35(7):1043–50.
Lai X, Zhao Y, Pan F, Yang B, Wang H, Wang S, He F. Enhanced optimal removal of nitrogen and organics from intermittently aerated vertical flow constructed wetlands: relative COD/N ratios and microbial responses. Chemosphere. 2020;244:125556. https://doi.org/10.1016/j.chemosphere.2019.125556.
Imfeld G, Braeckevelt M, Kuschk P, Richnow HH. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere. 2009;74(3):349–62.
Luo B, Ge Y, Han W, Fan X, Ren Y, Du Y, Shi M, Chang J. Decreases in ammonia volatilization in response to greater plant diversity in microcosms of constructed wetlands. Atmos Environ. 2016;142:414–9.
Di Luca GA, Maine MA, Mufarrege M, Hadad HR, Pedro MC, Sánchez GC, Caffaratti SE. Phosphorus distribution pattern in sediments of natural and constructed wetlands. Ecol Eng. 2017;108:227–233.
Xu D, Xu J, Wu J, Muhammad A. Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere. 2006;63(2):344–52.
Mathon B, Coquery M, Miège C, Vandycke A, Choubert J-M. Influence of water depth and season on the photodegradation of micropollutants in a free-water surface constructed wetland receiving treated wastewater. Chemosphere. 2019;235:260–70.
Schierano MC, Panigatti MC, Maine MA, Griffa CA, Boglione R. Horizontal subsurface flow constructed wetland for tertiary treatment of dairy wastewater: removal efficiencies and plant uptake. J Environ Manage. 2020;272:111094.
Dittrich E, Salamon-Albert É, Somfai D, Dolgos-Kovács A, Kiss T. Transpiration effect of Tufted sedge for a horizontal subsurface flow constructed wetland. Water Sci Technol. 2019;79(10):1905–11.
Zhang W, Guan A, Peng Q, Qi W,Qu J. Microbe-mediated simultaneous nitrogen reduction and sulfamethoxazole/N-acetylsulfamethoxazole removal in lab-scale constructed wetlands. Water Res. 2023:120233.
Wilson DB. Three microbial strategies for plant cell wall degradation. Ann N Y Acad Sci. 2008;1125(1):289–97.
Vicuña R. Bacterial degradation of lignin. Enzyme Microb Technol. 1988;10(11):646–55.
Sharma KM, Kumar R, Panwar S, Kumar A. Microbial alkaline proteases: optimization of production parameters and their properties. J Genet Eng Biotechnol. 2017;15(1):115–26.
Negi S. Lipases: A promising tool for food industry. Green Bio-processes: Enzyme Ind Food Process. 2019:181–198.
Gurung N, Ray S, Bose S, Rai V. A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. BioMed Res Int. 2013;2013.
Talamantes D, Biabini N, Dang H, Abdoun K, Berlemont R. Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol Biofuels. 2016;9:1–11.
Khan AA, Jilani G, Akhtar MS, Naqvi SS, Rasheed M. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci. 2009;1(1):48–58.
Zhu Y-G, Peng J, Chen C, **ong C, Li S, Ge A, Wang E,Liesack W. Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci. 2023.
Kertesz MA, Fellows E, Schmalenberger A. Rhizobacteria and plant sulfur supply. Adv Appl Microbiol. 2007;62:235–68.
Jun-**ng Y, Yong L, Zhi-Hong Y. Root-induced changes of pH, Eh, Fe (II) and fractions of Pb and Zn in rhizosphere soils of four wetland plants with different radial oxygen losses. Pedosphere. 2012;22(4):518–27.
Lamers LP, Van Diggelen JM, Op den Camp HJ, Visser EJ, Lucassen EC, Vile MA, Jetten MS, Smolders AJ, Roelofs JG. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: a review. Front Microbiol. 2012;3:156.
Wu H, Wang X, He X, Zhang S, Liang R, Shen J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci Total Environ. 2017;598:697–703.
Trias Mansilla R, Ruiz Rueda O, García Lledó A, Vilar Sanz A, López i Flores R, Quintana Pou X, Hallin S, Bañeras Vives L. Emergent macrophytes act selectively on ammonia-oxidizing bacteria and Archaea. © Appl Environ Microbiol. 2012;78(núm. 17):6352–6356.
Shen J-P, Zhang L-M, Di HJ, He J-Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front Microbiol. 2012;3:296.
Ji M, Hu Z, Hou C, Liu H, Ngo HH, Guo W, Lu S, Zhang J. New insights for enhancing the performance of constructed wetlands at low temperatures. Biores Technol. 2020;301:122722.
• Tang S, Liao Y, Xu Y, Dang Z, Zhu X, Ji G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: a review. Bioresour Technol. 2020;314:123759. This paper explains the microbial coupling mechanisms of nitrogen removal conserves oxygen and energy and the activity and diversity of nitrifiers and denitrifiers are affected significantly by plant species.
He S, Wang Y, Li C, Li Y, Zhou J. The nitrogen removal performance and microbial communities in a two-stage deep sequencing constructed wetland for advanced treatment of secondary effluent. Biores Technol. 2018;248:82–8.
Song S, Wang P, Liu Y, Zhao D, Leng X, An S. Effects of Oenanthe javanica on nitrogen removal in free-water surface constructed wetlands under low-temperature conditions. Int J Environ Res Public Health. 2019;16(8):1420.
Srithep P, Pornkulwat P, Limpiyakorn T. Contribution of ammonia-oxidizing archaea and ammonia-oxidizing bacteria to ammonia oxidation in two nitrifying reactors. Environ Sci Pollut Res. 2018;25:8676–87.
Rahman MM, Roberts KL, Grace MR, Kessler AJ, Cook PL. Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands. Sci Total Environ. 2019;666:608–17.
Kadlec RH. Phosphorus removal in emergent free surface wetlands. J Environ Sci Health. 2005;40(6–7):1293–306.
Du L, Chen Q, Liu P, Zhang X, Wang H, Zhou Q, Xu D, Wu Z. Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by integrated vertical-flow constructed wetlands (IVCWs). Biores Technol. 2017;243:204–11. https://doi.org/10.1016/j.biortech.2017.06.092.
Hei S, Xu H, Liu Y, Liu B, Zhang S, Zhu X, Lin W, Chen L, Jiang H, Cheng X, Yong X, Wu X, Huang X. Redox environment inducing strategy for enhancing biological phosphorus removal in a full-scale municipal wastewater treatment plant. J Clean Prod. 2022;376:134237. https://doi.org/10.1016/j.jclepro.2022.134237.
Rady MM, Taha SS, Kusvuran S. Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Sci Hortic. 2018;233:61–9.
Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y. Rock phosphate-solubilizing Actinomycetes: screening for plant growth-promoting activities. World J Microbiol Biotechnol. 2008;24(11):2565–75. https://doi.org/10.1007/s11274-008-9817-0.
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimarães CT, Schaffert RE, Sá NMH. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem. 2009;41(9):1782–7. https://doi.org/10.1016/j.soilbio.2008.01.012.
Xu F, Cao FQ, Kong Q, Zhou LL, Yuan Q, Zhu YJ, Wang Q, Du YD, Wang ZD. Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem Eng J. 2018;339:479–486. https://doi.org/10.1016/j.cej.2018.02.003.
Świątczak P, Cydzik-Kwiatkowska A. Performance and microbial characteristics of biomass in a full-scale aerobic granular sludge wastewater treatment plant. Environ Sci Pollut Res. 2018;25(2):1655–69. https://doi.org/10.1007/s11356-017-0615-9.
Wang Q, Ding J, **e H, Hao D, Du Y, Zhao C, Xu F, Kong Q, Wang B. Phosphorus removal performance of microbial-enhanced constructed wetlands that treat saline wastewater. J Clean Prod. 2021;288:125199.
Sun L, Zhao X, Zhang H, Zhang Y. Biological characteristics of a denitrifying phosphorus-accumulating bacterium. Ecol Eng. 2015;81:82–8. https://doi.org/10.1016/j.ecoleng.2015.04.030.
Rahman Z. An overview on heavy metal resistant microorganisms for simultaneous treatment of multiple chemical pollutants at co-contaminated sites, and their multipurpose application. J Hazard Mater. 2020;396:122682.
Yu G, Peng H, Fu Y, Yan X, Du C, Chen H. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Biores Technol. 2019;280:337–44.
Yu G, Wang G, Li J, Chi T, Wang S, Peng H, Chen H, Du C, Jiang C, Liu Y. Enhanced Cd2+ and Zn2+ removal from heavy metal wastewater in constructed wetlands with resistant microorganisms. Biores Technol. 2020;316:123898.
Huguenot D, Bois P, Cornu J, Jezequel K, Lollier M, Lebeau T. Remediation of sediment and water contaminated by copper in small-scaled constructed wetlands: effect of bioaugmentation and phytoextraction. Environ Sci Pollut Res. 2015;22:721–32.
Yu G, Wang G, Chi T, Du C, Wang J, Li P, Zhang Y, Wang S, Yang K, Long Y. Enhanced removal of heavy metals and metalloids by constructed wetlands: a review of approaches and mechanisms. Sci Total Environ. 2022:153516.
Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath M, Ahammad SZ. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Biores Technol. 2016;218:388–96.
Prum C, Dolphen R, Thiravetyan P. Enhancing arsenic removal from arsenic-contaminated water by Echinodorus cordifolius− endophytic Arthrobacter creatinolyticus interactions. J Environ Manage. 2018;213:11–9.
Ashraf S, Afzal M, Rehman K, Naveed M, Zahir ZA. Plant-endophyte synergism in constructed wetlands enhances the remediation of tannery effluent. Water Sci Technol. 2018;77(5):1262–70.
Hussain Z, Arslan M, Shabir G, Malik MH, Mohsin M, Iqbal S, Afzal M. Remediation of textile bleaching effluent by bacterial augmented horizontal flow and vertical flow constructed wetlands: a comparison at pilot scale. Sci Total Environ. 2019;685:370–9.
Izabel-Shen D, Li S, Luo T, Wang J, Li Y, Sun Q, Yu C-P, Hu A. Repeated introduction of micropollutants enhances microbial succession despite stable degradation patterns. ISME Commun. 2022;2(1):48.
Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36(10):2149–73.
McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394(6693):531–2.
Zhang L, Lyu T, Vargas CAR, Arias CA, Carvalho PN, Brix H. New insights into the effects of support matrix on the removal of organic micro-pollutants and the microbial community in constructed wetlands. Environ Pollut. 2018;240:699–708.
Lv T, Carvalho PN, Zhang L, Zhang Y, Button M, Arias CA, Weber KP, Brix H. Functionality of microbial communities in constructed wetlands used for pesticide remediation: influence of system design and sampling strategy. Water Res. 2017;110:241–51.
Zhang L, Lv T, Zhang Y, Stein OR, Arias CA, Brix H, Carvalho PN. Effects of constructed wetland design on ibuprofen removal – A mesocosm scale study. Sci Total Environ. 2017;609:38–45. https://doi.org/10.1016/j.scitotenv.2017.07.130.
Kurzbaum E, Kirzhner F, Armon R. Performance comparison of plant root biofilm, gravel attached biofilm and planktonic microbial populations, in phenol removal within a constructed wetland wastewater treatment system. Water Sa. 2016;42(1):166–70.
Author information
Authors and Affiliations
Contributions
SM drafted the manuscript and MB, SM, JF and VJ provided supervised SM and feedback. VJ revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moazzem, S., Bhuiyan, M., Muthukumaran, S. et al. Microbiome Wetlands in Nutrient and Contaminant Removal. Curr Pollution Rep 9, 694–709 (2023). https://doi.org/10.1007/s40726-023-00280-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-023-00280-9