Abstract
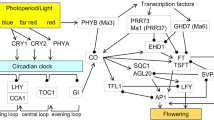
Flowering is a major factor that can decrease sugarcane productivity because it affects the quantity and quality of feedstock due to sucrose consumption from the stalk during inflorescence formation. Photoperiod is the main environmental factor involved in sugarcane floral induction, which occurs by the integration of gene regulatory networks in response to environmental and endogenous stimuli. One of the gene families involved in these regulatory networks are orthologs of the Arabidopsis thaliana FLOWERING LOCUS T (FT) gene, which encodes a phloem-mobile signal that stimulates floral induction at the shoot apical meristem. This work investigates homologs of the FT gene in sugarcane, as well as determines the putative function of these genes under inductive short-day photoperiods. We conducted in silico analyses to isolate putative FT orthologs in sugarcane and examined the expression levels of these genes under different photoperiodic conditions over a 24-hour-day-cycle in contrasting cultivars with different flowering responses. Three new FT orthologs were found with high similarity to FT homologs in other species. Among three genes identified, we focused on ScFT6, which has conserved amino acid sequence domains at characteristic positions for the flowering inducer of the phosphatidylethanolamine-binding protein (PEBP) gene family. ScFT6 expression varied according to diurnal variation and demonstrated a high transcriptional level under inductive shortening-day conditions. Cultivars with distinct flowering time responses display variable expression for the ScFT6. Therefore, ScFT6 is a putative orthologous gene in relation to FT that is controlled by photoperiod and diurnal regulation in sugarcane.






Similar content being viewed by others
References
Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel DJTEJ (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25(3):605–614
Aitken K, Berkman P, Rae A (2016) The first sugarcane genome assembly: how can we use it. Proc Aust Soc Sugar Cane Technol 38(1):193–199
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402
Araldi R, Silva FML, Ono EO, Rodrigues JD (2010) Flowering in sugarcane. Ciência Rural 40:694–702
Berding N, Hurney AP (2005) Flowering and lodging, physiological-based traits affecting cane and sugar yield: what do we know of their control mechanisms and how do we manage them? Field crops research 92(2–3):261–275
Berding N, Pendrigh RS, Dunne V (2010) Pursuing higher efficacy for managed photoperiodic initiation of sugarcane flowering in the tropics. Proc Aust Soc Sugar Cane Technol 2010(32):234–250
Bolger AM, Lohse M, Usadel BJB (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen EJN (1996) Control of inflorescence architecture in Antirrhinum. Nature 379(6568):791
Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen EJS (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275(5296):80–83
Burge C, Karlin SJJOM (1997) Prediction of complete gene structures in human genomic DNA. J Mol Biol 268(1):78–94
Coelho C, Costa Netto A, Colasanti J, Chalfun-Junior A (2013) A proposed model for the flowering signaling pathway of sugarcane under photoperiodic control. Genet Mol Res 12(2):1347–1359
Coelho CP, Minow MA, Chalfun-Júnior A, Colasanti J (2014) Putative sugarcane FT/TFL1 genes delay flowering time and alter reproductive architecture in Arabidopsis. Front Plant Sci 26(5):221
Corrêa LGG, Riaño-Pachón DM, Schrago CG, dos Santos RV, Mueller-Roeber B, Vincentz MMJPO (2008) The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PLoS ONE 3(8):e2944
Danilevskaya ON, Meng X, Hou Z, Ananiev EV, Simmons CR (2008) A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol 146(1):250–264
Dillewijn CV (1952) Botany of sugarcane: the chronica botanica. 617
Glassop D, Rae AL (2019) Expression of sugarcane genes associated with perception of photoperiod and floral induction reveals cycling over a 24-hour period. Funct Plant Biol 46(4):314–327
Goldemberg J (2007) Ethanol for a sustainable energy future. Science 315(5813):808–810
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29(7):644
Greenham K, McClung CR (2015) Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet 16(10):598
Grivet L, Arruda P (2002) Sugarcane genomics: depicting the complex genome of an important tropical crop. Curr Opin Plant Biol 5(2):122–127
Haas B, Papanicolaou A (2016) TransDecoder (find coding regions within transcripts).
Hanzawa Y, Money T, Bradley D (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci USA 102(21):7748–7753
Harig L, Beinecke FA, Oltmanns J, Muth J, Müller O, Rü** B, Twyman RM, Prüfer D, Noll GA (2012) Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J 72(6):908–921
Hayama R, Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol 135(2):677–684
Ho WWH, Weigel DJTPC (2014) Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. Plant Cell 26(2):552–564
Jaiswal D, De Souza AP, Larsen S, LeBauer DS, Miguez FE, Sparovek G, Buckeridge MS, Long SP (2017) Brazilian sugarcane ethanol as an expandable green alternative to crude oil use. Nat Clim Change 7(11):788–792
Júnior LCT, Marques MO, da Silva Neto HF, Camilotti F, Bernardi JH, Nogueira TAR (2009) Variação genotípica no florescimento, isoporização e características tecnológicas em seis cultivares de cana-de-açúcar/genotypic variation on the flower emission, pith process and technological characteristics in six sugarcane cultivars. Revista de Biologia e Ciências da Terra 9(1):12–18
Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel DJS (1999) Activation tagging of the floral inducer FT. Science 286(5446):1962–1965
Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz UJP (2011) Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol 156(4):1967–1977
Kim SY, Yu X, Michaels SD (2008) Regulation of CONSTANS and FLOWERING LOCUS T expression in response to changing light quality. Plant Physiol 148(1):269–279
Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286(5446):1960–1962
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43(10):1096–1105
Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135(4):767–774
Komiya R, Yokoi S, Shimamoto K (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136(20):3443–3450
Lazakis CM, Coneva V, Colasanti J (2011) ZCN8 encodes a potential orthologue of Arabidopsis FT florigen that integrates both endogenous and photoperiod flowering signals. J Exp Botany 62(14):4833–4842
Lebon G, Wojnarowiez G, Holzapfel B, Fontaine F, Vaillant-Gaveau N, Clément C (2008) Sugars and flowering in the grapevine (Vitis vinifera L.). J Exp Bot 59(10):2565–2578
Lin M-K, Belanger H, Lee Y-J, Varkonyi-Gasic E, Taoka K-I, Miura E, Xoconostle-Cázares B, Gendler K, Jorgensen RA, Phinney B (2007) FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 19(5):1488–1506
Ling H, Wu Q, Guo J, Xu L, Que Y (2014) Comprehensive selection of reference genes for gene expression normalization in sugarcane by real time quantitative RT-PCR. PLoS ONE 9(5):e97469
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Gee RC, He J, Bryant SH (20115) CDD: NCBI's conserved domain database. Nucleic Acids Res 43(1):222–226
Marques MO, Mutton MA, Nogueira TAR, Júnior LCT, de Nogueira GA, Bernardi JH (eds) (2008) Tecnologias na agroindústria canavieira Jaboticaba. FCAV, São Paulo, p. 319
Mattiello L, Riaño-Pachón DM, Martins MCM, da Cruz LP, Bassi D, Marchiori PER, Ribeiro PER, Labate RV, Labate MTV CA and, Menossi M (2015) Physiological and transcriptional analyses of developmental stages along sugarcane leaf. BMC Plant Biol 15(1):300
Melloni MLG, Melloni MNG, Scarpari MS, Garcia JC, Landell MG, Pinto LR (2015) Flowering of sugarcane genotypes under different artificial photoperiod conditions. Am J Plant Sci 6(03):456
Miller TA, Muslin EH, Dorweiler JE (2008) A maize CONSTANS-like gene, conz1, exhibits distinct diurnal expression patterns in varied photoperiods. Planta 227(6):1377–1388
Muszynski MG, Dam T, Li B, Shirbroun DM, Hou Z, Bruggemann E, Archibald R, Ananiev EV, Danilevskaya ON (2006) Delayed flowering1 encodes a basic leucine zipper protein that mediates floral inductive signals at the shoot apex in maize. Plant Physiol 142(4):1523–1536
Neto JVS, Gallo WL (2021) Potential impacts of vinasse biogas replacing fossil oil for power generation, natural gas, and increasing sugarcane energy in Brazil. Renew Sustain Energy Rev 135:110281
Ospina-Zapata DA, Madrigal Y, Alzate JF, Pabón-Mora N (2020) Evolution and expression of reproductive transition regulatory genes FT/TFL1 with emphasis in selected neotropical orchids. Front Plant Sci 11:469
Owczarzy R, Tataurov AV, Wu Y, Manthey JA, McQuisten KA, Almabrazi HG, Pedersen KF, Lin Y, Garretson J, McEntaggart NOJN (2008) IDT SciTools: a suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res 36(suppl2):W163–W169
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29(9):e45–e45
Pin PA, Benlloch R, Bonnet D, Wremerth-Weich E, Kraft T, Gielen JJ, Nilsson O (2010) An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330(6009):1397–1400
Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, Zamir D, Lifschitz EJD (1998) The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125(11):1979–1989
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland GJS (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288(5471):1613–1616
Shaw LM, Li C, Woods DP, Alvarez MA, Lin H, Lau MY, Chen A, Dubcovsky J (2020) Epistatic interactions between PHOTOPERIOD-1, CONSTANS 1 and CONSTANS 2 modulate the photoperiodic response in wheat. PLoS Genet 16(7):e1008812
Shim JS, Kubota A, Imaizumi T (2017) Circadian clock and photoperiodic flowering in Arabidopsis: CONSTANS is a hub for signal integration. Plant Physiol 173(1):5–15
Shrestha R, Gomez-Ariza J, Brambilla V, Fornara F (2014) Molecular control of seasonal flowering in rice, Arabidopsis and temperate cereals. Ann Bot 114(7):1445–1458
Singh P, Singh P, Singh J (2019) Effect of arrowing/flowering on juice quality of sugarcane. Indian J Sugarcane Technol 34(02):82–84
Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol 66:441–464
Srivastava RP, Singh SP, Singh PRATAP, Singh SB (2006) Artificial induction of flowering in sugarcane under sub-tropical conditions—a successful approach. Sugar Tech 8(2–3):184–186
Steibel JP, Poletto R, Coussens PM, Rosa GJ (2009) A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics 94(2):146–152
Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410(6832):1116
Takada S, Goto K (2003) TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15(12):2856–2865
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316(5827):1033–1036
Tamura K, Dudley J, Nei M, Kumar SJMB (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599
Taoka KI, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki SJN (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476(7360):332
Telles GP, Braga MD, Dias Z, Tzy-Li L, Quitzau JA, Silva FRD, Meidanis J (2001) Bioinformatics of the sugarcane EST project. Genet Mol Biol 24(1–4):9–15
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22(22):4673–4680
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303(5660):1003–1006
Venail J, da Silva Santos PH, Manechini JR, Alves LC, Scarpari M, Falcão T, Romanel E, Brito M, Vicentini R, Pinto L, Jackson SD (2022) Analysis of the PEBP gene family and identification of a novel FLOWERING LOCUS T orthologue in sugarcane. J Exp Bot 73(7):2035–2049
Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feiljohn R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339:704–707
Wickland DP, Hanzawa YJMP (2015) The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: functional evolution and molecular mechanisms. Mol Plant 8(7):983–997
Wolabu TW, Zhang F, Niu L, Kalve S, Bhatnagar-Mathur P, Muszynski MG, Tadege M (2016) Three FLOWERING LOCUS T‐like genes function as potential florigens and mediate photoperiod response in sorghum. New Phytol 210(3):946–959
Acknowledgements
This research was funded by CNPq (The Brazilian National Council for Scientific and Technological Development grant #437647/2018-8 and #310216/2019-2) and CAPES (Brazilian federal government agency under the Ministry of Education).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40626_2023_276_MOESM1_ESM.docx
Supplementary Fig. 1 Day length throughout the experiment period according to each treatment. The plants were acclimated for 20 days at greenhouse with shade netting of 50% spacing. Two light treatments were imposed on the sugarcane plants for 30 days, with a daily reduction of 1 min. Leaf samples were collected after 30 days. Supplementary material 1 (DOCX 23.5 kb)
40626_2023_276_MOESM2_ESM.docx
Supplementary Fig. 2 Conserved PEBP domain present in sugarcane FT and TFL putative orthologs: ScFT6 (a), ScTFL3 (b) and ScTFL4 (c). Query seq. indicates the length of the query sequence by the number of amino acids. Small triangles indicate the amino acid involved in conserved features/sites, such as catalytic and binding sites. Data generated from < https://www.ncbi.nlm.nih.gov/>. Supplementary material 2 (DOCX 90.6 kb)
40626_2023_276_MOESM3_ESM.docx
Supplementary Fig. 3 Fold-change estimates for the ScFT6 gene expression over 24-hours. The log2 fold-changes for four contrasts (abscissa) are presented. Interval bars indicate the 95% confidence interval. Comparisons between times of day whose confidence interval includes the value 0 (1 in fold-change scale) are not significant at α = 5%. Supplementary material 3 (DOCX 72.1 kb)
40626_2023_276_MOESM4_ESM.docx
Supplementary Fig. 4 Fold-change estimates for the ScFT6 gene expression under two contrasting photoperiod (Shortening-day—Lengthening-day). The log2 fold-changes for two contrasts (abscissa) are presented. Interval bars indicate the 95% confidence interval. Comparisons between photoperiods whose confidence interval includes the value 0 (1 in fold-change scale) are not significant at α = 5%. Supplementary material 4 (DOCX 18.1 kb)
40626_2023_276_MOESM5_ESM.docx
Supplementary Fig. 5 Mean stalk diameter, ASD, (a) and mean stalk height, MSH, (b) of cultivars RB 85-5453 (flowering) and CTC 9003 (non-flowering). The symbol (*) indicates statistical difference between means by F test at p ≤ 0.05. The vertical bars indicate the standard errors of the mean. Supplementary material 5 (DOCX 74.8 kb)
40626_2023_276_MOESM6_ESM.docx
Supplementary Fig. 6 Percentage of dead leaves (% DF, left) and leaf area (LA, right) of cultivars RB 85-5453 and CTC 9003. The symbol (*) indicates statistical difference between means by F test at p ≤ 0.05. Supplementary material 6 (DOCX 76.1 kb)
40626_2023_276_MOESM7_ESM.docx
Supplementary Fig. 7 Fold-change estimates for the ScFT6 gene expression in two sugarcane contrasting cultivars, under two contrasting photoperiod (Shortening-day—Lengthening-day). The log2 fold-changes for three contrasts (abscissa) are presented. Interval bars indicate the 95% confidence interval. Comparisons between cultivars whose confidence interval includes the value 0 (1 in fold-change scale) are not significant at α = 5%. Supplementary material 7 (DOCX 94.3 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Linhares-Neto, M.V., Schumacher, P.V., Ribeiro, T.H.C. et al. Molecular screening reveals a photoperiod responsive floral regulator in sugarcane. Theor. Exp. Plant Physiol. 35, 199–214 (2023). https://doi.org/10.1007/s40626-023-00276-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-023-00276-2