Abstract
Background and objectives
Highly effective vaccines against severe acute respiratory syndrome virus 2 have been developed and administered worldwide. However, protection from coronavirus disease 2019 is not absolute and an optimal vaccination regimen needs to be established. This study assessed the clinical efficacy of the coronavirus disease 2019 vaccine among dialysis patients receiving 3 or 4 doses of vaccine.
Design, setting, participants, and measurements
This retrospective study was conducted using the electronic database of Clalit Health Maintenance Organization in Israel. Chronic dialysis patients treated with either hemodialysis or peritoneal dialysis during the coronavirus disease 2019 pandemic were included. We compared clinical outcomes of patients who had received three or four doses of the severe acute respiratory syndrome virus 2 vaccine.
Results
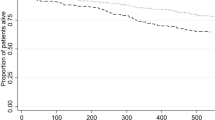
This study included 1,030 patients on chronic dialysis, with a mean age of 68 ± 13 years. Among them, 502 patients had received 3 doses of the vaccine and 528 received 4 doses. Severe acute respiratory syndrome virus 2 infection rates, severe COVID-19 that resulted in hospitalizations, COVID-19–related mortality and all-cause mortality rates were lower among chronic dialysis patients who received a fourth dose of vaccine as compared to those who received only 3 doses (after controlling for age, sex and comorbidities). Despite lower mortality rates observed with the Omicron variant, the fourth dose was significantly associated with reduced COVID-19-related mortality (1.7% vs. 3.8%, p = 0.04). Odds ratio for COVID-19-related mortality was 0.44 with 95% CI 0.2–0.98.
Conclusions
As seen in the general population and with previous vaccine boosters, the fourth dose of the BNT162b2 vaccine reduced rates of severe COVID-19-related hospitalization and mortality among chronic dialysis patients. Further studies are needed to establish the optimal regimens of vaccination for patients on chronic dialysis.
Graphical abstract





Similar content being viewed by others
Data availability
Data access requests can be adressed to the corresponding author.
References
Hsu CM, Weiner DE, Aweh G et al (2021) COVID-19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. https://doi.org/10.1053/j.ajkd.2021.01.003
Hilbrands LB, Duivenvoorden R, Vart P et al (2020) Erratum: COVID-19–related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 35(11):1973–1983. https://doi.org/10.1093/ndt/gfaa261
Wand O, Nacasch N, Fadeela A et al (2022) Humoral response and breakthrough infections with SARS-CoV-2 B.1.617.2 variant in vaccinated maintenance hemodialysis patients. J Nephrol 35(5):1479–1487. https://doi.org/10.1007/s40620-022-01245-9
Nacasch N, Cohen-Hagai K, Tayar N et al (2022) Humoral response to hepatitis B and COVID-19 vaccine among maintenance hemodialysis patients. Vaccines (Basel). https://doi.org/10.3390/vaccines10101670
Grupper A, Sharon N, Finn T et al (2021) Humoral response to the pfizer bnt162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 16:1037–1042. https://doi.org/10.2215/CJN.03500321
Nacasch N, Erez D, Lishner M et al (2022) Long-term antibody response to the BNT162b2 vaccine among maintenance hemodialysis patients. Am J Kidney Dis 79(1):137–139
Shashar M, Nacasch N, Grupper A et al (2022) Humoral response to Pfizer BNT162b2 vaccine booster in maintenance hemodialysis patients. Am J Nephrol. https://doi.org/10.1159/000521676
Bar-On YM, Goldberg Y, Mandel M et al (2022) Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. https://doi.org/10.1056/nejmoa2201570
Magen O, Waxman JG, Makov-Assif M et al (2022) Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. https://doi.org/10.1056/NEJMOA2201688/SUPPL_FILE/NEJMOA2201688_DISCLOSURES.PDF
Einbinder Y, Perl J, Nacasch N et al (2022) Humoral response and SARS-CoV-2 infection risk following the third and fourth doses of the BNT162b2 vaccine in dialysis patients. Am J Nephrol 53:586–590. https://doi.org/10.1159/000525309
Housset P, Kubab S, Hanafi L et al (2022) Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int 101:1289–1290. https://doi.org/10.1016/J.KINT.2022.04.006
COVID-19 Treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed 27 Nov 2022
Goldberg Y, Mandel M, Bar-On YM et al (2021) Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. https://doi.org/10.1056/NEJMOA2114228
Chemaitelly H, Tang P, Hasan MR et al (2021) Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. medRxiv. https://doi.org/10.1101/2021.08.25.21262584
Syed AM, Ciling A, Taha TY et al (2022) Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2200592119
Callaway E (2022) Why does the Omicron sub-variant spread faster than the original? Nature. https://doi.org/10.1038/d41586-022-00471-2
Panizo N, Albert E, Giménez-Civera E et al (2022) Dynamics of SARS-CoV-2-Spike-reactive antibody and T-cell responses in chronic kidney disease patients within 3 months after COVID-19 full vaccination. Clin Kidney J 15:1562–1573. https://doi.org/10.1093/CKJ/SFAC093
Barh D, Tiwari S, Rodrigues Gomes LG et al (2022) SARS-CoV-2 variants show a gradual declining pathogenicity and pro-inflammatory cytokine stimulation, an increasing antigenic and anti-inflammatory cytokine induction, and rising structural protein instability: a minimal number genome-based approach. Inflammation. https://doi.org/10.1007/S10753-022-01734-W
Acknowledgements
We thank Faye Schreiber, MS for editing the manuscript. She is an employee of Meir Medical Center.
Funding
This article did not receive any financial support.
Author information
Authors and Affiliations
Contributions
Research area and study design: KC-H, TH-L, SB, MS; Data acquisition: NN, SB, KC-H; Data analysis and interpretation: KC-H, TH-L, YE, AG, SB, KC-H; Statistical analysis: KC-H, TH-L; Supervision or mentorship: OW, SB, KC-H, MS. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or other conflicts of interest to declare.
Ethical approval
The study was approved by the Ethics Committee and Institutional Review Board of Meir Medical Center (MMC 220–21). The committee waived the requirement for informed consent due to the retrospective nature of the study. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cohen-Hagai, K., Hornik-Lurie, T., Benchetrit, S. et al. Clinical efficacy of the fourth dose of the BNT162b2 vaccine in maintenance dialysis patients . J Nephrol 36, 1957–1964 (2023). https://doi.org/10.1007/s40620-023-01667-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01667-z