Abstract
Background
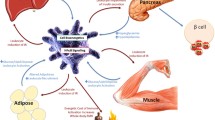
Obesity promotes cellular immunometabolism changes that trigger the activation of macrophages and lymphocytes, leading to systemic inflammation. Activated leukocytes undergo metabolic reprogramming, increasing glycolytic activity.
Objective
To examine whether the reduction in the inflammatory state associated with bariatric surgery is associated with decreased glycolytic activity in leukocytes.
Setting
Single-center, prospective observational study.
Methods
This study involved 18 patients with obesity undergoing bariatric surgery. All measurements were performed preoperatively and six months postoperatively. Peripheral blood mononuclear cells and plasma were obtained to determine the glycolytic rate and mitochondrial membrane potential as surrogates of the metabolic switching and high-sensitivity C-reactive protein, adipokines, and CD69 expression as inflammatory and activation markers.
Results
Glycolytic activity engaged by CD3/CD28 activation was reduced six months after bariatric surgery, associated with decreased levels of T helper (Th) 1 and Th17 signature cytokines. An overall reduction in inflammatory markers was observed, which correlated with a higher adiponectin/leptin ratio.
Conclusions
Metabolic and bariatric surgery-induced weight loss leads to reprogramming in T cells’ metabolic machinery, resulting in reduced stimulation of glycolysis after activation, which may explain the decrease in systemic inflammation mediated by cytokines such as interferon-γ and interleukin-17A.





Similar content being viewed by others
References
Wang Q, Wu H (2018) T Cells in adipose tissue: critical players in immunometabolism. Front Immunol. https://doi.org/10.3389/fimmu.2018.02509
Fuster JJ, Ouchi N, Gokce N, Walsh K (2016) Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res 118:1786–1807. https://doi.org/10.1161/CIRCRESAHA.115.306885
McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q et al (2012) B lymphocytes in human subcutaneous adipose crown-like structures. Obes Silver Spring Md 20:1372–1378. https://doi.org/10.1038/oby.2012.54
Kuroda M, Sakaue H (2017) Adipocyte death and chronic inflammation in obesity. J Med Investig JMI 64:193–196. https://doi.org/10.2152/jmi.64.193
Belizário JE, Faintuch J, Garay-Malpartida M (2018) Gut microbiome dysbiosis and immunometabolism: new frontiers for treatment of metabolic diseases. Mediators Inflamm 2018:2037838. https://doi.org/10.1155/2018/2037838
Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O et al (2004) Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord J Int Assoc Study Obes 28:674–679. https://doi.org/10.1038/sj.ijo.0802609
Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH et al (2008) Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 103:467–476. https://doi.org/10.1161/CIRCRESAHA.108.177105
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF et al (1950) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol Baltim Md 2011(186):3299–3303. https://doi.org/10.4049/jimmunol.1003613
Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR et al (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208:1367–1376. https://doi.org/10.1084/jem.20110278
Franchi L, Monteleone I, Hao L-Y, Spahr MA, Zhao W, Liu X et al (1950) Inhibiting oxidative phosphorylation in vivo restrains Th17 effector responses and ameliorates murine colitis. J Immunol Baltim Md 2017(198):2735–2746. https://doi.org/10.4049/jimmunol.1600810
Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ (1950) Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol Baltim Md 2014(192):136–144. https://doi.org/10.4049/jimmunol.1301158
Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC et al (2016) Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol 46:1970–1983. https://doi.org/10.1002/eji.201545861
Surendar J, Frohberger SJ, Karunakaran I, Schmitt V, Stamminger W, Neumann A-L et al (2019) Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front Immunol 10:2555. https://doi.org/10.3389/fimmu.2019.02555
Nicholas DA, Proctor EA, Agrawal M, Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L et al (2019) Fatty acid metabolites combine with reduced β oxidation to activate Th17 inflammation in human Type 2 diabetes. Cell Metab 30:447-461 e5. https://doi.org/10.1016/j.cmet.2019.07.004
Frühbeck G, Catalán V, Rodríguez A, Ramírez B, Becerril S, Salvador J et al (2019) Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients. https://doi.org/10.3390/nu11020454
Unamuno X, Izaguirre M, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Becerril S et al (2019) Increase of the adiponectin/leptin ratio in patients with obesity and Type 2 Diabetes after Roux-en-Y gastric bypass. Nutrients. https://doi.org/10.3390/nu11092069
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883
Andersson DP, Wahrenberg H, Toft E, Qvisth V, Löfgren P, Hertel K et al (2014) Waist circumference to assess reversal of insulin resistance following weight reduction after bariatric surgery: cohort and cross-sectional studies. Int J Obes 38:438–443. https://doi.org/10.1038/ijo.2013.88
Menk AV, Schar** NE, Moreci RS, Zeng X, Guy C, Salvatore S et al (2018) Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep 22:1509–1521. https://doi.org/10.1016/j.celrep.2018.01.040
Datta K, Lauritzen MH, Merchant M, Jang T, Liu S-C, Hurd R et al (2019) Reversed metabolic reprogramming as a measure of cancer treatment efficacy in rat C6 glioma model. PLoS ONE 14:e0225313. https://doi.org/10.1371/journal.pone.0225313
Marin E, Bouchet-Delbos L, Renoult O, Louvet C, Nerriere-Daguin V, Managh AJ et al (2019) Human tolerogenic dendritic cells regulate immune responses through lactate synthesis. Cell Metab 30:1075-1090 e8. https://doi.org/10.1016/j.cmet.2019.11.011
Kohlgruber AC, LaMarche NM, Lynch L (2016) Adipose tissue at the nexus of systemic and cellular immunometabolism. Semin Immunol 28:431–440. https://doi.org/10.1016/j.smim.2016.09.005
Lee C-F, Lo Y-C, Cheng C-H, Furtmüller GJ, Oh B, Andrade-Oliveira V et al (2015) Preventing allograft rejection by targeting immune metabolism. Cell Rep 13:760–770. https://doi.org/10.1016/j.celrep.2015.09.036
Yin Y, Choi S-C, Xu Z, Perry DJ, Seay H, Croker BP et al (2015) Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7:274ra18. https://doi.org/10.1126/scitranslmed.aaa0835
Madsen EL, Rissanen A, Bruun JM, Skogstrand K, Tonstad S, Hougaard DM et al (2008) Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol 158:179–187. https://doi.org/10.1530/EJE-07-0721
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC et al (2016) Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23:591–601. https://doi.org/10.1016/j.cmet.2016.02.005
Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD et al (2009) Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 360:859–873. https://doi.org/10.1056/NEJMoa0804748
Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS, Weidenbacher HJ et al (2016) Bariatric surgery and long-term durability of weight loss. JAMA Surg 151:1046–1055. https://doi.org/10.1001/jamasurg.2016.2317
Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO et al (2011) Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obes Silver Spring Md 19:1987–1998. https://doi.org/10.1038/oby.2011.230
Askarpour M, Khani D, Sheikhi A, Ghaedi E, Alizadeh S (2019) Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and meta-analysis. Obes Surg 29:2631–2647. https://doi.org/10.1007/s11695-019-03926-0
Lord GM, Matarese G, Howard JK, Bloom SR, Lechler RI (2002) Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol 72:330–338
Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V (2000) Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 199:15–24. https://doi.org/10.1006/cimm.1999.1594
Viardot A, Heilbronn LK, Samocha-Bonet D, Mackay F, Campbell LV, Samaras K (2012) Obesity is associated with activated and insulin resistant immune cells: immune cell insulin resistance. Diabetes Metab Res Rev 28:447–454. https://doi.org/10.1002/dmrr.2302
Viardot A, Lord RV, Samaras K (2010) The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J Clin Endocrinol Metab 95:2845–2850. https://doi.org/10.1210/jc.2009-2371
Samaras K, Viardot A, Botelho NK, Jenkins A, Lord RV (2013) Immune cell-mediated inflammation and the early improvements in glucose metabolism after gastric banding surgery. Diabetologia 56:2564–2572. https://doi.org/10.1007/s00125-013-3033-7
Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS (2010) T cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obes Silver Spring Md 18:1918–1925. https://doi.org/10.1038/oby.2010.1
Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G et al (1996) Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol 26:659–668. https://doi.org/10.1002/eji.1830260323
Chang C-H, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D et al (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153:1239–1251. https://doi.org/10.1016/j.cell.2013.05.016
Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO (2016) Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354:481–484. https://doi.org/10.1126/science.aaf6284
Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ et al (2015) Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep 12:1042–1055. https://doi.org/10.1016/j.celrep.2015.07.014
Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K et al (2014) De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 20:1327–1333. https://doi.org/10.1038/nm.3704
Klein Geltink RI, Kyle RL, Pearce EL (2018) Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol 36:461–488. https://doi.org/10.1146/annurev-immunol-042617-053019
Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA et al (2013) Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38:225–236. https://doi.org/10.1016/j.immuni.2012.10.020
Buck MD, O’Sullivan D, Pearce EL (2015) T cell metabolism drives immunity. J Exp Med 212:1345–1360. https://doi.org/10.1084/jem.20151159
Monzo-Beltran L, Vazquez-Tarragón A, Cerdà C, Garcia-Perez P, Iradi A, Sánchez C et al (2017) One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol 12:389–402. https://doi.org/10.1016/j.redox.2017.02.003
Villarreal-Calderón JR, Cuéllar RX, Ramos-González MR, Rubio-Infante N, Castillo EC, Elizondo-Montemayor L et al (2019) Interplay between the adaptive immune system and insulin resistance in weight loss induced by bariatric surgery. Oxid Med Cell Longev 2019:3940739. https://doi.org/10.1155/2019/3940739
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G et al (2015) Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet 386:964–973. https://doi.org/10.1016/S0140-6736(15)00075-6
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD et al (2014) Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 370:2002–2013. https://doi.org/10.1056/NEJMoa1401329
Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA et al (2015) Three-year outcomes of bariatric surgery vs lifestyle intervention for Type 2 Diabetes Mellitus treatment: a randomized clinical trial. JAMA Surg 150:931. https://doi.org/10.1001/jamasurg.2015.1534
Iannelli A, Anty R, Schneck AS, Tran A, Gugenheim J (2011) Inflammation, insulin resistance, lipid disturbances, anthropometrics, and metabolic syndrome in morbidly obese patients: a case control study comparing laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. Surgery 149:364–370. https://doi.org/10.1016/j.surg.2010.08.013
Coupaye M, Rivière P, Breuil MC, Castel B, Bogard C, Dupré T et al (2014) Comparison of nutritional status during the first year after sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Surg 24:276–283. https://doi.org/10.1007/s11695-013-1089-6
Kokkinos A, Liaskos C, Alexiadou K, Papassotiriou I, Margeli A, Argyrakopoulou G et al (2020) Plasma levels of soluble urokinase plasminogen activator receptor (suPAR) and high-sensitivity C-reactive protein after Roux-en-Y gastric bypass or sleeve gastrectomy: a 1-year prospective observational study. J Endocrinol Invest. https://doi.org/10.1007/s40618-020-01358-7
The Look AHEAD Research Group (2014) Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study: 8-year weight losses in look AHEAD. Obesity 22:5–13. https://doi.org/10.1002/oby.20662
Burguera B, Jesús Tur J, Escudero AJ, Alos M, Pagán A, Cortés B et al (2015) An intensive lifestyle intervention is an effective treatment of morbid obesity: the TRAMOMTANA study—a two-year randomized controlled clinical trial. Int J Endocrinol 2015:1–11. https://doi.org/10.1155/2015/194696
Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ et al (2016) Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 315:2424. https://doi.org/10.1001/jama.2016.7602
Lee B-C, Lee J (2014) Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta BBA Mol Basis Dis 1842:446–462. https://doi.org/10.1016/j.bbadis.2013.05.017
Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E et al (2014) Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 20:614–625. https://doi.org/10.1016/j.cmet.2014.08.010
Acknowledgements
The authors would like to thank Dr. Eduardo Vázquez-Garza for their excellent technical assistance in flow cytometry and Lucia Villareal for their help with anthropometry measurements.
Funding
This work was partially supported by Cardiovascular Medicine Research Group- Tecnologico de Monterrey 0020CAT131 as well as CONACYT-México grants 151136 and 256577 (G. G-Rivas) and by the XIGNUX foundation 00220CIE241 (LE-M). JR V-C was supported by the Graduate Student Fellowship of CONACYT. This work was submitted in partial fulfillment of the requirements for the Master’s degree (JR V-C) for the Master in Biomedical Sciences at Tecnologico de Monterrey.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jose Romeo Villarreal-Calderón, Elena Cristina Castillo, Ricardo X. Cuellar-Tamez, Manuel García-Garza, Leticia Elizondo-Montemayor, and Gerardo García-Rivas declare that they have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Villarreal-Calderón, J.R., Castillo, E.C., Cuellar-Tamez, R.X. et al. Reduced Th1 response is associated with lower glycolytic activity in activated peripheral blood mononuclear cells after metabolic and bariatric surgery. J Endocrinol Invest 44, 2819–2830 (2021). https://doi.org/10.1007/s40618-021-01587-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01587-4