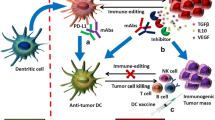
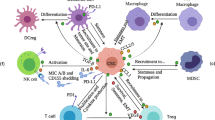
Abstract
Purpose of Review
Cancer immune evasion is still a major obstacle in elimination of cancer cells and current cancer treatment. This review focuses on immune evasion mechanisms at each step of the cancer immune cycle to better understand the escape of the tumor cells from the immune system and to provide insight to guide the design of effective anticancer therapeutic strategies.
Recent Findings
There are a number of factors that contribute to immune escape, including restriction of antigen recognition, inhibition of immune system cells, immunosuppressive effects, and accumulation of specific metabolites in TME. However, identification of immune escape mechanisms in cancer still remains an active area of research, as both tumor and immune response in individuals are heterogeneous and diverse.
Summary
A better understanding of immune escape mechanisms in cancer can provide the development of clinically applicable therapeutic options in a range of cancers and contribute to the management of the disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. https://doi.org/10.1146/annurev.immunol.22.012703.104803.
Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. https://doi.org/10.1111/j.1365-2567.2007.02587.x.
• Tang S, Ning Q, Yang L, Mo Z, Tang S. Mechanisms of immune escape in the cancer immune cycle. Int Immunopharmacol. 2020;86:106700. https://doi.org/10.1016/j.intimp.2020.106700. This review discussed the mechanisms of immune escape in the cancer immune cycle and therapeutic strategies for overcoming immune escape. This paper was integral to the current study.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;25;39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. https://doi.org/10.1038/nri.2016.107.
Maleki VS. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):157. https://doi.org/10.1186/s40425-018-0479-.
•• Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–98. https://doi.org/10.1016/j.semcancer.2015.03.004. This paper outlined approaches that aim to increase tumor-killing activities by identifying immunosuppressive factors for successful cancer treatment. This paper was integral to the current study.
Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–608. https://doi.org/10.1158/1535-7163.MCT-17-0386.
Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20(7):398–411. https://doi.org/10.1038/s41568-020-0263-0.
Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;4;536(7614):91–5. https://doi.org/10.1038/nature18945.
Wang BD, Lee NH. Aberrant RNA splicing in cancer and drug resistance. Cancers (Basel). 2018;20;10(11):458. https://doi.org/10.3390/cancers10110458.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov;2015;5(12):1282–95. https://doi.org/10.1158/2159-8290.CD-15-1020.
Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol. 1997;34(16–17):1157–65. https://doi.org/10.1016/s0161-5890(97)00144-2.
Song MK, Park BB, Uhm JE. Resistance mechanisms to CAR T-cell therapy and overcoming Strategy in B-cell hematologic malignancies. Int J Mol Sci. 2019;10;20(20):5010. https://doi.org/10.3390/ijms20205010.
Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol. 2019;113:31–7. https://doi.org/10.1016/j.molimm.2018.03.025.
• Marciscano AE, Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol. 2021;52: 101481. https://doi.org/10.1016/j.smim.2021.101481. This review outlined the role of dendritic cells in cancer and cancer therapies. This paper was integral to the current study.
Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6(6):476–83. https://doi.org/10.1038/nri1845.
Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83(5):451–61. https://doi.org/10.1111/j.1440-1711.2005.01371.x.
Abu N, Rus Bakarurraini NAA, Nasir SN. Extracellular vesicles and DAMPs in cancer: a mini-review. Front Immunol. 2021;15(12): 740548. https://doi.org/10.3389/fimmu.2021.740548.
Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT, Storkus WJ, Whiteside TL. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res. 2002;8:1787–93.
Lissoni P, Vigore L, Ferranti R, et al. Circulating dendritic cells in early and advanced cancer patients: diminished percent in the metastatic disease. J Biol Regul Homeost Agents. 1999;13:216–9.
Zeid NA, Muller HK. S100 positive dendritic cells in human lung tumors associated with cell differentiation and enhanced survival. Pathology. 1993;25:338–43.
•• Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front Pharmacol. 2022;24(13): 868695. https://doi.org/10.3389/fphar.2022.868695. In this study, the effects of the TME on cancer cells and immune cells, and anti-cancer strategies were reviewed. This paper was integral to the current study.
Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–7. https://doi.org/10.1016/j.coi.2014.12.009.
Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–19. https://doi.org/10.1016/j.molmed.2007.12.007.
You Z, Chi H. Lipid metabolism in dendritic cell biology. Immunol Rev. 2023;317(1):137–51. https://doi.org/10.1111/imr.13215.
Cycon KA, Rimsza LM, Murphy SP. Alterations in CIITA constitute a common mechanism accounting for downregulation of MHC class II expression in diffuse large B-cell lymphoma (DLBCL). Exp Hematol. 2009;37(2):184–94. https://doi.org/10.1016/j.exphem.2008.10.001.
Bushway M, Cycon KA, Mulvaney K, Murphy SP. Coordinate loss of MHC class II expression in the diffuse large B cell lymphoma cell line OCI-Ly2 is due to a novel mutation in RFX-AP. Immunogenetics. 2010;62(2):109–16. https://doi.org/10.1007/s00251-009-0418-3.
Garrido F, Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res. 2001;83:117–58. https://doi.org/10.1016/s0065-230x(01)83005-0.
Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. https://doi.org/10.1016/j.coi.2017.01.002.
Lee HG, Cho MJ, Choi JM. Bystander CD4+ T cells: crossroads between innate and adaptive immunity. Exp Mol Med. 2020;52(8):1255–63. https://doi.org/10.1038/s12276-020-00486-7.
Jeong S, Park SH. Co-stimulatory receptors in cancers and their implications for cancer immunotherapy. Immune Netw. 2020;7;20(1):e3. https://doi.org/10.4110/in.2020.20.e3.
de Winde CM, Munday C, Acton SE. Molecular mechanisms of dendritic cell migration in immunity and cancer. Med Microbiol Immunol. 2020;209(4):515–29. https://doi.org/10.1007/s00430-020-00680-4.
Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20(5):432–47. https://doi.org/10.1038/s41423-023-00990-6.
Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol. 2018;15(4):346–52. https://doi.org/10.1038/s41423-018-0005-3.
Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–36. https://doi.org/10.1016/j.ccell.2016.06.003.
Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2(7):585–9. https://doi.org/10.1038/89726.
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;9;523(7559):231–5. https://doi.org/10.1038/nature14404
Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;6;192(9):1213–22. https://doi.org/10.1084/jem.192.9.1213.
Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–91. https://doi.org/10.1517/14728222.11.11.1473.
Hobbs SJ, Nolz JC. Regulation of T cell trafficking by enzymatic synthesis of O-glycans. Front Immunol. 2017;8:600. https://doi.org/10.3389/fimmu.2017.00600.
•• Duru G, van Egmond M, Heemskerk N. A window of opportunity: targeting cancer endothelium to enhance immunotherapy. Front Immunol. 2020;11: 584723. https://doi.org/10.3389/fimmu.2020.584723. This paper reviewed views and strategies targeting the tumor vasculature to increase the infiltration of cytotoxic immune cells during vascularization, thereby increasing the efficacy of immunotherapy. This paper was integral to the current study.
Karin N. CXCR3 ligands in cancer and autoimmunity, chemoattraction of effector t cells, and beyond. Front Immunol. 2020;11:976. https://doi.org/10.3389/fimmu.2020.00976.
Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554–61. https://doi.org/10.1182/blood.v98.13.3554.
Langenes V, Svensson H, Börjesson L, Gustavsson B, Bemark M, Sjöling Å, et al. Expression of the chemokine decoy receptor D6 is decreased in colon adenocarcinomas. Cancer Immunol Immunother. 2013;62:1687–95. https://doi.org/10.1007/s00262-013-1472-0.
Yu KD, Wang X, Yang C, Zeng XH, Shao ZM. Host genotype and tumor phenotype of chemokine decoy receptors integrally affect breast cancer relapse. Oncotarget. 2015;6:26519–27. https://doi.org/10.18632/oncotarget.4470.
Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. https://doi.org/10.1084/jem.20101956.
Apte RS, Chen DS, Ferrara N VEGF in signaling and disease: beyond discovery and development. Cell. 2019;7;176(6):1248–1264. https://doi.org/10.1016/j.cell.2019.01.021.
Zarychta E, Ruszkowska-Ciastek B. Cooperation between angiogenesis, vasculogenesis, chemotaxis, and coagulation in breast cancer metastases development: pathophysiological point of view. Biomedicines. 2022;27;10(2):300. https://doi.org/10.3390/biomedicines10020300.
Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–15. https://doi.org/10.1038/nm.3541.
Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–26. https://doi.org/10.1002/eji.200324270.
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers. 2015;7(4):2443–58. https://doi.org/10.3390/cancers7040902.
Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2(3):187–93. https://doi.org/10.1158/2326-6066.CIR-14-0002.
Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26(6):878–85. https://doi.org/10.1038/s41591-020-0880-x.
Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20. https://doi.org/10.1016/j.it.2016.01.004.
Petty AJ, Owen DH, Yang Y, Huang X. Targeting tumor-associated macrophages in cancer ımmunotherapy. Cancers (Basel). 2021;22;13(21):5318. https://doi.org/10.3390/cancers1
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci USA. 2018;115:E4041–50. https://doi.org/10.1073/pnas.1720948115.
• Taylor BC, Balko JM. Mechanisms of MHC-I downregulation and role in immunotherapy response. Front Immunol. 2022;28(13): 844866. https://doi.org/10.3389/fimmu.2022.844866. This paper outlined current research in MHC-I downregulation and its impact on immunotherapy response in patients. This paper was integral to the current study.
Park HS, Cho U, Im SY, Yoo CY, Jung JH, Suh YJ, et al. Loss of human leukocyte antigen class I expression is associated with poor prognosis in patients with advanced breast cancer. J Pathol Transl Med. 2019;53:75–85. https://doi.org/10.4132/jptm.2018.10.11.
Lobashevsky AL, Krueger-Sersen M, Britton RM, Littrell CA, Singh S, Cui CP, et al. Pretransplant HLA ty** revealed loss of heterozygosity in the major histocompatibility complex in a patient with acute myeloid leukemia. Hum Immunol. 2019;80:257–62. https://doi.org/10.1016/j.humimm.2019.02.009.
Bernal M, García-Alcalde F, Concha A, Cano C, Blanco A, Garrido F, et al. Genome-wide differential genetic profiling characterizes colorectal cancers with genetic instability and specific routes to HLA class I loss and immune escape. Cancer Immunol Immunother. 2012;61:803–16. https://doi.org/10.1007/s00262-011-1147-7.
Saleh MH, Wang L, Goldberg MS. Improving cancer immunotherapy with DNA methyltransferase inhibitors. Cancer Immunol Immunother. 2016;65:787–96. https://doi.org/10.1007/s00262-015-1776-3.
Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–32. https://doi.org/10.1038/sj.onc.1210610.
Lazaridou MF, Gonschorek E, Massa C, Friedrich M, Handke D, Mueller A, et al. Identification of miR-200a-5p targeting the peptide transporter TAP1 and its association with the clinical outcome of melanoma patients. OncoImmunology. 2020;9:1774323. https://doi.org/10.1080/2162402X.2020.1774323.
Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. New Eng J Med. 2016;375(18):1767–78. https://doi.org/10.1056/NEJMra1514296.
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;1;111(7):3635–43. https://doi.org/10.1182/blood-2007-11-123141
Liu Q, Qi Y, Zhai J, Kong X, Wang X, Wang Z, et al. Molecular and clinical characterization of LAG3 in breast cancer through 2994 samples. Front Immunol. 2021;29(12): 599207. https://doi.org/10.3389/fimmu.2021.599207.
Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;10;176(1–2):334–347.e12. https://doi.org/10.1016/j.cell.2018.11.010.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–185. https://doi.org/10.1038/s41577-019-0224-6.
Mulati K, Hamanishi J, Matsumura N, Chamoto K, Mise N, Abiko K, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120(1):115–27. https://doi.org/10.1038/s41416-018-0313-5.
Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8(1): e000911. https://doi.org/10.1136/jitc-2020-000911.
Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156(1):74–85. https://doi.org/10.1111/imm.13001.
Nakano M, Ito M, Tanaka R, Yamaguchi K, Ariyama H, Mitsugi K, et al. PD-1+ TIM-3+ T cells in malignant ascites predict prognosis of gastrointestinal cancer. Cancer Sci. 2018;109(9):2986–92. https://doi.org/10.1111/cas.13723.
Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;24;7(8):e1466769. https://doi.org/10.1080/2162402X.2018.1466769
•• **a L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;5;20(1):28. https://doi.org/10.1186/s12943-021-01316-8. This paper discussed how metabolic reprogramming of cancer cells and immune cells regulate antitumor immune response. This paper was integral to the current study.
Badur MG, Metallo CM. Reverse engineering the cancer metabolic network using flux analysis to understand drivers of human disease. Metab Eng. 2018;45:95–108. https://doi.org/10.1016/j.ymben.2017.11.013.
Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;6;25(6):1282–1293.e7. https://doi.org/10.1016/j.cmet.2016.12.018
Watson MJ, Delgoffe GM. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J Clin Invest. 2022;18;132(2):e148549. https://doi.org/10.1172/JCI148549.
Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379–401. https://doi.org/10.1038/s41573-019-0016-5.
Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;2;11(1):100. https://doi.org/10.1186/s13045-018-0644-y
Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, et al. IDO1 and kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Cancer Res. 2019;15;79(6):1138–1150. https://doi.org/10.1158/0008-5472.CAN-18-0668
Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell. 2018;12;33(3):480–494.e7. https://doi.org/10.1016/j.ccell.2018.02.005
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. https://doi.org/10.1038/nri2506.
Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25. https://doi.org/10.1038/s41416-018-0333-1.
Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Front Oncol. 2021;11(11): 610303. https://doi.org/10.3389/fonc.2021.610303.
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;15;64(16):5839–49. https://doi.org/10.1158/0008-5472.CAN-04-0465.
• Feng Y, Ye Z, Song F, He Y, Liu J. The role of TAMs in tumor microenvironment and new research progress. Stem Cells Int. 2022;2022(15):5775696. https://doi.org/10.1155/2022/5775696. This article provides a theoretical basis for the functions of TAM and finding a potentially effective macrophage-targeted tumor therapy. This paper was integral to the current study.
Tao J, Ji F, Wang F, Liu B, Zhu Y. Neuroprotective effects of progranulin in ischemic mice. Brain Res. 2012;3(1436):130–6. https://doi.org/10.1016/j.brainres.2011.11.063.
Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–80.
Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35.
Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–9. https://doi.org/10.1111/cas.14069.
Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46. https://doi.org/10.1016/j.immuni.2007.08.014.
Matoba T, Imai M, Ohkura N, Kawakita D, Ijichi K, Toyama T, et al. Regulatory T cells expressing abundant CTLA-4 on the cell surface with a proliferative gene profile are key features of human head and neck cancer. Int J Cancer. 2019;1;144(11):2811–2822. https://doi.org/10.1002/ijc.32024.
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18(12):1332–41. https://doi.org/10.1038/ni.3868.
Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307. https://doi.org/10.1038/s41577-019-0257-x.
Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;1;19(21):5994–6005. https://doi.org/10.1158/1078-0432.CCR-12-3497
Willsmore ZN, Harris RJ, Crescioli S, Hussein K, Kakkassery H, Thapa D, et al. B Cells in patients with melanoma: ımplications for treatment with checkpoint ınhibitor antibodies. Front Immunol. 2021;25(11): 622442. https://doi.org/10.3389/fimmu.2020.622442.
Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 2022;15(1):61. https://doi.org/10.1186/s13045-022-01282-8.
Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63(1):113–24. https://doi.org/10.1016/j.molimm.2014.02.020.
Author information
Authors and Affiliations
Contributions
AC contributed to the entire manuscript
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caner, A. Immune Escape Mechanism of Cancer. Curr Mol Bio Rep 10, 9–19 (2024). https://doi.org/10.1007/s40610-023-00157-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-023-00157-2