Abstract
Background and Aims
Ischemia following acute myocardial infarction (AMI) increases the level of pro-fibrotic and inflammatory cytokines, including transforming growth factor (TGF)-β and tumor necrosis factor (TNF)-α. N-acetylcysteine (NAC) has therapeutic benefits in the management of patients with AMI. To the best of our knowledge, this is the first study that has evaluated the effect of NAC on TNF-α and TGF-β levels in patients with AMI.
Methods
Following confirmation of AMI, 88 patients were randomly administered NAC 600 mg (Fluimucil®, Zambon, Ticino, Switzerland) or placebo orally twice daily for 3 days. For quantification of TGF-β and TNF-α serum levels after 24 and 72 h of NAC or placebo administration, peripheral venous blood (10 mL) samples were collected at these time points.
Results
Comparisons between levels of TGF-β and TNF-α after 24 and 72 h within the NAC or placebo groups revealed that there was not any significant difference except for TGF-β levels in the placebo group, which increased significantly over time (p = 0.042). Significant relationships existed between patients’ ejection fraction (p = 0.005) and TGF-β levels.
Conclusions
Receiving NAC could prevent TGF-β levels from increasing after 72 h as compared with not receiving NAC. As TGF-β had strong correlations with the ejection fraction, its antagonism seems to be important in the prevention of remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acute myocardial infarction (AMI) triggers an ischemic state in the myocardium, after which a process of remodeling is initiated by gradual myocardial ventricular dilation, hypertrophy, and distortion of left ventricular (LV) geometry [1]. The remodeling process, which can be categorized into the two phases of early (≤72 h) and late (>72 h) [2], is considered to be a determinant of mortality and morbidity in patients after AMI [3]. Several mechanisms contribute to the remodeling process, including myocardial cell death, fibrotic changes in cardiomyocytes following collagen synthesis, and inflammation due to increased expression of pro-inflammatory cytokines [4–6].
Ischemia following AMI provoked an increase in the level of main pro-fibrotic cytokine, transforming growth factor (TGF)-β, which induces fibrotic depositions in the cardiomyocytes [7]. TGF-β plays a significant role in the pathogenesis of the remodeling process, as its inhibition in the proliferative phase of remodeling can prevent the LV from hypertrophy and decrease the extent of fibrosis in the non-infarcted segments of the myocardium and improve LV geometry [8, 9].
On the other hand, AMI is associated with acute up-regulation of pro-inflammatory cytokines, with tumor necrosis factor (TNF)-α being the most important [10]. TNF-α stimulated the remodeling process and provoked myocardial dysfunction after AMI [11–13]. Moreover, TNF-α can increase the expression of angiotensin receptor in the cardiac fibroblasts of animal models, which increased the activity of angiotensin and therefore induced fibrotic changes [14].
N-acetylcysteine (NAC), previously recognized as an acetaminophen antidote, has been used in different clinical situations because of its antioxidant, free radicals scavenger, and glutathione-replenishing properties [15]. NAC is hypothesized to have numerous therapeutic benefits in the management of cardiovascular diseases, including post-AMI cardiac remodeling [16–18]. In animal models of ischemia and reperfusion, NAC decreased infarct size [19, 20]. In combination with thrombolytics, NAC reduced oxidative stress, induced a trend toward more rapid reperfusion, and enhanced preservation of LV function [21, 22].
Although glutathione is considered to have a major role in preserving body homeostasis and protecting cells against toxic agents, it is not transported well into cells due to its large molecular size. Moreover, l-cysteine, the amino acid involved in the intracellular synthesis of glutathione, is toxic to humans. NAC can easily be deacetylated in cells to provide l-cysteine and therefore increase the intracellular glutathione concentration. Glutathione is a necessary factor for the activation of T lymphocytes and polymorphonuclear leukocytes in addition to cytokine production [23]. As nuclear factor (NF)-κB has a role in the inducible transcription of TNF-α and oxidative stress can induce its nuclear translocation, antioxidants including NAC can act as potent inhibitors of NF-κB activation [24, 25]. This may be the explanation behind how NAC might prevent the production of TNF-α. With respect to TGF-β, NAC can change this cytokine to its biologically inactive form and inhibit its binding to the receptor [26]. On the other hand, fibronectin, a glycoprotein involved in tissue remodeling, can be released in response to a variety of cytokines including TGF-β as its strongest stimulator. Therefore, by inhibiting the TGF-β-induced fibronectin production, NAC can be effective in blocking tissue remodeling [27].
To the best of our knowledge, this is the first study evaluating the effect of NAC on TNF-α and TGF-β levels in human subjects with AMI to investigate whether NAC might be beneficial in reducing remodeling.
2 Methods
This randomized double-blind clinical trial (registration no.: IRCT201102283449N5 at http://www.irct.ir) was conducted at the Tehran Heart Centre, one of the referral teaching hospitals for cardiovascular disorders in Tehran, Iran from August 2010 to August 2011.
The sample size of the study (44 patients in each group) was calculated based on the change in the serum TNF-α concentration following NAC administration [11]. The power of the study was considered to be 95 % (α = 0.05 and β = 0.20).
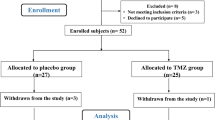
After obtaining written informed consent, patients fulfilling diagnostic criteria for ST-segment elevation myocardial infarction (STEMI) were included in the study. However, patients with pre-hospital delays of more than 12 h, previous AMI, heart failure, atrial fibrillation, or chronic diseases, in addition to those under treatment with anti-inflammatory agents or antioxidants, were not eligible for the present study. The local ethical committee approved this trial and the investigation conforms to the principles outlined in the Declaration of Helsinki. Following confirmation of STEMI, patients were randomly administered NAC effervescent tablet 600 mg (Fluimucil®, Zambon, Ticino, Switzerland) or placebo together with their standard treatment twice daily for 3 days. The pharmacotherapy management of all patients was the same, including aspirin, clopidogrel, captopril, metoprolol, nitrate, and high-dose atorvastatin (80 mg).
We documented data regarding patients’ demography, past medical and drug history, laboratory parameters, ischemic time [defined as the time from symptom onset to their management either by thrombolytic therapy or primary percutaneous coronary intervention (P-PCI)], type of management (thrombolytic or P-PCI), and echocardiographic and coronary angiographic findings (number of arteries affected) if evaluated. Echocardiography was performed for all patients before discharge.
For quantification of TGF-β and TNF-α serum levels 24 and 72 h after NAC or placebo administration, peripheral venous blood (10 mL) samples were collected at these time points. Samples were centrifuged at 3,000 rpm for 10 min, and serums were separated and stored at −70 °C. Serum levels of TGF-β and TNF-α were measured using commercial ELISA kits (Bender MedSystems, Vienna, Austria).
2.1 Statistical Analysis
Data were analyzed using SPSS® (version 16) statistical software. We reported categorical variables as frequency counts and percentages while continuous variables were summarized as medians and ranges or means and standard deviations. For assessing the normal distribution of variables, the Kolmogorov–Smirnov test was used. The associations of TGF-β and TNF-α serum levels with patients’ characteristics were investigated using the chi-square statistical test or Fisher’s exact test for discrete variables and the Mann–Whitney test for continuous variables. Spearman correlation coefficient was used to evaluate the correlation between continuous variables. A generalized estimating equation was used to estimate the correlation between repeated biomarker levels. Log-transformation was performed for non-normally distributed variables where applicable. We used two independent samples t tests to compare levels of log-transformed TGF-β and TNF-α between NAC and placebo groups. A paired t test was used to compare these biomarkers’ log-transformed levels in the NAC and placebo groups individually.
3 Results
3.1 Comparisons Between Patients in the N-Acetylcysteine and Placebo Groups
This prospective study was conducted on 88 patients who fulfilled the inclusion criteria of the trial. The age range of the studied population was 40–92 years and 72 (82 %) were males. Patients’ demographic data, including age, sex, past medical and drug history, ischemic time as well as the number of subjects receiving P-PCI, did not have a statistically significant difference in the NAC versus placebo groups (Table 1). Furthermore, the comparison of biomarker levels measured after 24 (TGF-β-24 h, TNF-α-24 h) and 72 (TGF-β-72 h, TNF-α-72 h) hours did not show any statistically significant difference between the NAC and placebo groups (Table 2). Comparisons between levels of TGF-β and TNF-α after 24 and 72 h within the NAC or placebo groups revealed that there was not any significant difference except for TGF-β levels in the placebo group, which increased significantly with time passed (p = 0.042) [Fig. 1]. Parallel comparisons were made using the log-transformed levels of these biomarkers with similar results.
3.2 Associations of Biomarker Levels with Patient Characteristics
We did not find any significant relationship between levels of TGF-β-24 h, TNF-α-24 h, TGF-β-72 h, or TNF-α-72 h and patients’ demographic data, including age and sex. Hypertension or hyperlipidemia did not have any associations with biomarkers’ levels. However, significant relationships existed between past medical history of diabetes mellitus and TGF-β-72 h (p = 0.033). Moreover, drug history of statins (HMG-CoA reductase inhibitors) had a significant association with TGF-β-72 h (p = 0.009). Being a smoker had a relationship with the level of TNF-α-24 h (p = 0.049). There was not any association between type of reperfusion management and biomarker levels, while coronary angiographic findings showed a statistically significant relationship with the TGF-β-72 h level (p = 0.014). The level of TGF-β-72 h had a statistically significant difference between patients with two-vessel disease and those with left main coronary artery (LMCA) disease (p = 0.001). Moreover, significant differences existed between patients with triple-vessel disease and those with LMCA disease (p = 0.021) as the latter had higher levels of TGF-β-72 h. In evaluating associations of echocardiographic findings and biomarker levels, significant relationships existed between ejection fraction and TGF-β-72 h (p = 0.005) as well as between intraventricular septum abnormality and TNF-α-24 h (p = 0.038).
3.3 Correlations Between Biomarker Levels and Patient Characteristics
We found significant correlations between the level of TNF-α-24 h and TGF-β-72 h (r = 0.231, p = 0.03). Significant correlation existed between the level of TNF-α-72 h and glycosylated hemoglobin (HbA1c) serum level (r = 0.655, p = 0.029). The level of TGF-β-24 h had significant correlations with ischemic time (r = −0.233, p = 0.037) as well as cardiac troponin T levels of patients within 6 h of admission (r = 0.218, p = 0.042), white blood cell (WBC) count (r = 0.358, p = 0.001) and ALT serum levels (r = 0.377, p = 0.048). Finally, significant correlations existed between TGF-β-72 h levels and matrix metalloproteinase (MMP)-9 measured after 72 h (r = 0.330, p = 0.003) in addition to patients’ ejection fraction (r = −0.311, p = 0.009).
4 Discussion
Several physiologic pathways including inflammation and fibrosis may involve in the pathogenesis of post-myocardial infarction (MI) structural changes called remodeling. As TNF-α and TGF-β are known to be the major biomarkers that contribute to each of these mentioned mechanisms and NAC is proposed to have beneficial effects in acute cardiology, in this study we evaluated the impact of NAC on these biomarkers. TNF-α tends to peak within 24 h following MI, and decreased toward baseline 3 days after MI [28]. Although the TNF-α level trend was in favor of those who received NAC, the difference was not significant between groups.
While we could not find any significant effect on the TNF-α level, NAC could prevent TGF-β from increasing. In patients who received placebo the level of TGF-β increased significantly after 72 h; however, this significant elevation did not take place in patients in the NAC group. Antagonism of TGF-β can lead to two opposite effects depending on the time. Early TGF-β inhibition, within the first 24 h after AMI, can increase levels of pro-inflammatory cytokines and infiltration of neutrophils, and consequently intensify the expression of MMPs which may result in aggravation of LV dysfunction and increase the rate of mortality [8]. Conversely, TGF-β antagonism after this time can have beneficial effects by reducing the extent of fibrotic and hypertrophic changes in the myocardium [9, 29, 30]. In the present study, we found that NAC did not have any significant effect on the level of TGF-β at 24 h, the time at which its inhibition can have a detrimental outcome. However, NAC administration could prevent TGF-β from increasing at 72 h as compared with patients receiving placebo, the time at which the proliferative phase of remodeling will start, and therefore its suppression could have favorable therapeutic effects. Higher serum concentrations of TGF-β had strong positive correlations with LV systolic function and patients’ ejection fraction in the present study, which showed that a relationship existed between TGF-β and cardiac remodeling. This finding puts more emphasis on the necessity of TGF-β inhibition to prevent cardiac remodeling and its untoward consequences. As TGF-β was shown to promote extracellular matrix synthesis and collagen crosslink took place after MI, it could have an important role in the signaling pathway of LV remodeling [31]. An increased TGF-β level after MI was associated with the development of heart failure secondary to cardiac remodeling [31].
In the present study, a significant association was found between serum concentrations of TGF-β and the presence of diabetes. This finding is in line with a previous study, which showed a relationship between elevated serum concentrations of TGF-β and diabetes after considering demographic, anthropometric, metabolic, and lifestyle factors [32]. This could be explained by the mechanism of insulin resistance as inflammation can be an important factor in its development and thus the incidence of diabetes [33].
Another association was between a history of statin use and the level of TGF-β. TGF-β is one of the most important mediators of cardiomyocyte fibrosis and hypertrophic growth through the action of Smad proteins as an essential component of the intracellular signaling pathway [34]. Statins can suppress the up-regulation of TGF-β induced by angiotensin and the resultant cardiac remodeling and systolic dysfunction [35, 36]. This suppression can be attributed to the inhibition of superoxide production favored by angiotensin [36]. Thus, the low level of TGF-β in patients receiving statins as observed in the present study is a reasonable finding.
The other finding of this study was the relationship between the coronary angiography finding, in particular stenosis of the LMCA, and TGF-β levels. Although acute STEMI following LMCA stenosis is not prevalent (0.8–5.4 %), as reported in previous studies, its mortality rate is very high despite emergent P-PCI [37–40]. The association of TGF-β levels with severity of coronary artery disease (CAD) has not been consistent among previous studies. A positive relationship was seen between the severity of CAD and TGF-β levels in the Wang et al. [41] study, as was seen in our study. In contrast, Grainger et al. [42] reported lower serum concentrations of TGF-β in patients with severe CAD. Despite having positive atherosclerosis plaque stabilization effects [43], TGF-β can lead to accumulation of extracellular matrix by decreasing the production of collagenase and promotion of atherosclerosis through increasing the collagen synthesis [44].
Ischemic time and cardiac troponin C levels were other factors that had correlations with the level of TGF-β. This could show the importance of acceleration in the reperfusion management of patients with STEMI in order to reduce the extent of remodeling. Furthermore, the correlation of cardiac troponin with TGF-β levels revealed that the extension of myocardial necrosis had a positive relationship with the degree of cardiac remodeling.
Strong positive correlations existed between WBC counts and TGF-β levels. Due to the inflammatory state in patients with STEMI, an increase in the number of WBCs occurs [45]. The association of TGF-β with inflammatory status is further elucidated with the link that existed between TGF-β and TNF-α in this study. In previous studies, associations of WBCs with ejection fraction as a marker of systolic function and LV remodeling have been reported [46]. As TGF-β is a biomarker of remodeling, the positive correlation between its level and WBCs seems rational. Furthermore, in a study by Walshe et al. [47], inhibition of TGF-β led to a reduction in WBC adhesion to endothelial cells and an increase in the WBC count, which could be another potential explanation for this correlation.
With respect to TNF-α we observed higher levels of this cytokine in patients who smoke than in non-smokers, which is in line with a previous study on patients with chronic obstructive pulmonary disease [48]. In contrast, some other studies did not find a significant difference in the level of TNF-α in smokers versus non-smokers [49, 50]. Higher levels of TNF-α in patients with AMI who smoke in the present study can develop the hypothesis that smoking can be the stimulus of enhanced systemic inflammation and potentially higher extension of remodeling.
A significant positive correlation existed between the levels of TNF-α and HbA1c. As TNF-α contributed to the insulin resistance in patients with diabetes [51], its high level can lead to poor glycemic control in this population and, consequently, raised HbA1c. In concordance with this statement, patients with concomitant rheumatoid arthritis or Crohn’s disease and diabetes who received anti-TNF-α therapeutic agents had significant improvements in their HbA1c, which reduced from 6.5 to 5.5 % after treatment.
Our study was not without limitations. Also, NAC is known to reduce oxidative stress but we did not evaluate its efficacy by measuring oxidative products. Moreover, NAC was administered orally in this study. As enrolled patients were suffering from STEMI and therefore hypoperfusion, this may lead to a decrease in the possible effects of NAC. As another limitation of this study, we did not follow up our patients in order to assess the long-term effects of NAC, in particular on echocardiography parameters. Furthermore, we did not use magnetic resonance imaging for the evaluation of remodeling in our patients, which may reduce the precision of interpretation of our findings.
5 Conclusion
This is the first study to evaluate the possible effects of NAC on TGF-β and TNF-α levels in patients admitted with STEMI. Administration of NAC could prevent TGF-β levels from increasing after 72 h as compared with patients who received placebo. As TGF-β had a strong correlation with ejection fraction as a marker of LV systolic function its late antagonism seems to be important. Elucidating the role of NAC in the prevention of cardiac remodeling post-AMI merits further larger clinical trials.
References
Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72.
Sutton MJ, Sharpe N. Left ventricular remodeling after myocardial infarction. Pathophysiology and therapy. Circulation. 2000;101:2981–8.
Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–63.
Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47.
Suematsu N, Tsutsui H, Wen J, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–23.
Hori M, Nishida K. Oxidative stress and left ventricular remodeling after myocardial infarction. Cardiovasc Res. 2009;81:457–64.
Vilahur G, Juan-Babot O, Pena E, et al. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol. 2011;50:522–33.
Ikeuchi M, Tsutsui H, Shiomi T, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64:526–35.
Okada H, Takemura G, Kosai K, et al. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–7.
Murray DB, Levick SP, Brower GL, Janicki JS. Inhibition of matrix metalloproteinase activity prevents increases in myocardial tumor necrosis factor-α. J Mol Cell Cardiol. 2010;49:245–50.
Bourraindeloup M, Adamy C, Candiani G, et al. N-acetylcysteine treatment normalizes serum tumor necrosis factor α level and hinders the progression of cardiac injury in hypertensive rats. Circulation. 2004;110:2003–9.
Skyschally A, Gres P, Hoffmann S, et al. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–6.
Thielmann M, Dorge H, Martin C, et al. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002;90:807–13.
Peng J, Gurantz D, Tran V, Cowling RT, Greenberg BH. Tumor necrosis factor-alpha-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosis. Circ Res. 2002;91:1119–26.
De Vries N, De Flora S. N-acetyl-l-cysteine. J Cell Biochem Suppl. 1994;17F:270–7.
Sochman J. N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know?! J Am Coll Cardiol. 2002;39:1422–8.
Talasaz AH, Khalili H, Fahimi F, Salarifar M. Potential role of N-acetylcysteine in cardiovascular disorders. Therapy. 2011;8:237–45.
Adamy C, Mulder P, Khouzami L, et al. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J Mol Cell Cardiol. 2007;43:344–53.
Meyer M, LeWinter MM, Bell SP, et al. N-acetylcysteine-enhanced contrast provides cardiorenal protection. JACC Cardiovasc Interv. 2009;2:215–21.
Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K. Comparison of the protective effect of N-acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. J Pharmacol Sci. 2008;106:571–7.
Arstall MA, Yang J, Stafford I, Betts WH, Horowitz JD. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation. 1995;92:2855–62.
Yesilbursa D, Serdar A, Senturk T, et al. Effect of N-acetylcysteine on oxidative stress and ventricular function in patients with myocardial infarction. Heart Vessels. 2006;21:33–7.
Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Pharmacother. 2003;57:145–55.
Ullmann KS, Northrop JP, Verweij CL, Crabtree GR. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–52.
Cu A, Ye Q, Sarria R, et al. N-acetylcysteine inhibits TNF-alpha, sTNFR, and TGF-beta1 release by alveolar macrophages in idiopathic pulmonary fibrosis in vitro. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:147–54.
Meurer SK, Lahme B, Tihaa L, Weiskirchen R, Gressner AM. N-acetyl-l-cysteine suppresses TGF-b signaling at distinct molecular steps: the biological efficacy of a multifunctional, antifibrotic drug. Biochem Pharmacol. 2005;70:1026–34.
Sugiura H, Ichikawa T, Liu X, et al. N-acetyl-l-cysteine inhibits TGF-beta1-induced profibrotic responses in fibroblasts. Pulm Pharmacol Ther. 2009;22:487–91.
Zhang Y, Zhao J, Lau WB, et al. Tumor necrosis factor-α and lymphotoxin-α mediate myocardial ischemic injury via TNF receptor 1, but are cardioprotective when activating TNF receptor 2. PLoS One. 2013;8:e60227.
Panek AN, Posch MG, Alenina N, et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS One. 2009;4:e6743.
Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–58.
Stefanon I, Valero-Muñoz M, Fernandes AA, et al. Left and right ventricle late remodeling following myocardial infarction in rats. PLoS One. 2013;8:e64986.
Herder C, Zierer A, Koenig W, et al. Transforming growth factor-beta1 and incident type 2 diabetes: results from the MONICA/KORA case-cohort study, 1984-2002. Diabetes Care. 2009;32:1921–3.
Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48:1038–50.
Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44.
Schultz Jel J, Witt SA, Glascock BJ, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–96.
Yagi S, Aihara K, Ikeda Y, et al. Pitavastatin, an HMG-CoA reductase inhibitor, exerts eNOS-independent protective actions against angiotensin II induced cardiovascular remodeling and renal insufficiency. Circ Res. 2008;102:68–76.
Lee SW, Hong MK, Lee CW, et al. Early and late clinical outcomes after primary stenting of the unprotected left main coronary artery stenosis in the setting of acute myocardial infarction. Int J Cardiol. 2004;97:73–6.
Tang HC, Wong A, Wong P, et al. Clinical features and outcome of emergency percutaneous intervention of left main coronary artery occlusion in acute myocardial infarction. Singap Med J. 2007;48:1122–4.
Neri R, Migliorini A, Moschi G, et al. Percutaneous reperfusion of left main coronary disease complicated by acute myocardial infarction. Catheter Cardiovasc Interv. 2002;56:31–4.
Valeur N, Gaster AL, Saunamaki K. Percutaneous revascularization in acute myocardial infarction due to left main stem occlusion. Scand Cardiovasc J. 2005;39:24–9.
Wang XL, Liu SX, Wilcken DE. Circulating transforming growth factor beta 1 and coronary artery disease. Cardiovasc Res. 1997;34:404–10.
Grainger DJ, Kemp PR, Metcalfe JC, et al. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med. 1995;1:74–9.
Cipollone F, Fazia M, Mincione G, et al. Increased expression of transforming growth factor-β1 as a stabilizing factor in human atherosclerotic plaques. Stroke. 2004;35:2253–7.
Aihara K, Ikeda Y, Yagi S, Akaike M, Matsumoto T. Transforming growth factor-β1 as a common target molecule for development of cardiovascular diseases, renal insufficiency and metabolic syndrome. Cardiol Res Pract. 2010;2011:175381.
Di Stefano R, Di Bello V, Barsotti MC, et al. Inflammatory markers and cardiac function in acute coronary syndrome: difference in ST-segment elevation myocardial infarction (STEMI) and in non-STEMI models. Biomed Pharmacother. 2009;63:773–80.
Smit JJ, Ottervanger JP, Slingerland RJ, On-TIME Study Group, et al. Comparison of usefulness of C-reactive protein versus white blood cell count to predict outcome after primary percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol. 2008;101:446–51.
Walshe TE, Dole VS, Maharaj A, et al. Inhibition of VEGF or TGF-β signaling activates endothelium and increases leukocyte rolling. Arterioscler Thromb Vasc Biol. 2009;29:1185–92.
Tanni SE, Pelegrino NR, Angeleli AY, Correa C, Godoy I. Smoking status and tumor necrosis factor-alpha mediated systemic inflammation in COPD patients. J Inflamm (Lond). 2010;7:29.
Diez-Pina JM, Fernandez-Aceñero MJ, Llorente-Alonso MJ, et al. Tumor necrosis factor alpha as a marker of systemic and local inflammation in “healthy” smokers. Int J Gen Med. 2009;2:9–14.
Vernooy JH, Küςükaycan M, Jacobs J, et al. Local and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;186:1218–24.
Gupta-Ganguli M, Cox K, Means B, Gerling I, Solomon SS. Does therapy with anti-TNF-alpha improve glucose tolerance and control in patients with type 2 diabetes? Diabetes Care. 2011;34:e121.
Funding
This study was awarded a grant from the Tehran University of Medical Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Registration no.: IRCT201102283449N5 at http://www.irct.ir.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Talasaz, A.H., Khalili, H., Jenab, Y. et al. N-Acetylcysteine Effects on Transforming Growth Factor-β and Tumor Necrosis Factor-α Serum Levels as Pro-Fibrotic and Inflammatory Biomarkers in Patients Following ST-Segment Elevation Myocardial Infarction. Drugs R D 13, 199–205 (2013). https://doi.org/10.1007/s40268-013-0025-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-013-0025-5