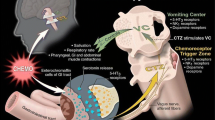
Abstract
Chemotherapy-induced nausea and vomiting (CINV) negatively affects adherence to treatment and quality of life in older patients, and should be managed differently due to physiological and/or psychological changes; also consider impacts of patient comorbidities and polypharmacy. Prevent CINV using serotonin 3-receptor antagonists, neurokinin 1-receptor antagonists, corticosteroids and other drug classes (dopamine 2,3-receptor antagonists should mainly be used as a rescue antiemetic), and simplify CINV prophylaxis as much as possible. Further data is needed for CINV prophylaxis in older patients for optimal guideline-directed treatment.

Similar content being viewed by others
References
Piechotta V, Adams A, Haque M, et al. Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis. Cochrane Database Syst Rev. 2021;11(11):CD012775.
Herrstedt J, Lindberg S, Petersen PC. Prevention of chemotherapy-induced nausea and vomiting in the older patient: optimizing outcomes. Drugs Aging. 2022;39(1):1–21.
Majem M, de las Peñas R, Virizuela JA, et al. SEOM clinical guideline update for the prevention of chemotherapy-induced nausea and vomiting (2021). Clin Transl Oncol. 2022;24(4):712–23.
Chow R, Valdez C, Chow N, et al. Oral cannabinoid for the prophylaxis of chemotherapy-induced nausea and vomiting—a systematic review and meta-analysis. Support Care Cancer. 2020;28(5):2095–103.
Moertel C, Reitemeier RJ. Controlled clinical studies of orally administered antiemetic drugs. Gastroenterology. 1969;57(3):262–8.
Jolliet P, Nion S, Allain-Veyrac G, et al. Evidence of lowest brain penetration of an antiemetic drug, metopimazine, compared to domperidone, metoclopramide and chlorpromazine, using an in vitro model of the blood-brain barrier. Pharmacol Res. 2007;56(1):11–7.
Moertel C, Reitemeier RJ. Controlled studies of metopimazine for the treatment of nausea and vomiting. J Clin Pharmacol. 1973;13(7):283–7.
Herrstedt J, Sigsgaard T, Boesgaard M, et al. Ondansetron plus metopimazine compared with ondansetron alone in patients receiving moderately emetogenic chemotherapy. N Engl J Med. 1993;328(15):1076–80.
Roscoe JA, Heckler CE, Morrow GR, et al. Prevention of delayed nausea: a University Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. J Clin Oncol. 2012;30(27):3389–95.
Gralla RJ, Itri L, Pisko SE, et al. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J med. 1981;305(16):905–9.
Huys J. Cytostatic-associated vomiting effectively inhibited by domperidone (R 33 812). Cancer Chenother Pharmacol. 1978;1(4):215–8.
Cubeddu LX, Hoffman IS, Fuenmayor NT, et al. Antagonism of serotonin S3 receptors with ondansetron prevents nausea and emesis induced by cyclophosphamide-containing chemotherapy regimens. J Clin Oncol. 1990;8(10):1721–7.
Cubeddu LX, Hoffmann IS, Fuenmayor NT, et al. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med. 1990;322(12):810–6.
Marty M, Pouillart P, Scholl S, et al. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med. 1990;322(12):816–21.
Jones AL, Hill AS, Soukop M, et al. Comparison of dexamethasone and ondansetron in the prophylaxis of emesis induced by moderately emetogenic chemotherapy. Lancet. 1991;338(8765):483–7.
Warr D, Willan A, Fine S, et al. Superiority of granisetron to dexamethasone plus prochlorperazine in the prevention of chemotherapy-induced emesis. J Natl Cancer Inst. 1991;83(16):1169–73.
Warr D, Wilan A, Venner P, et al. A randomised, double-blind comparison of granisetron with high-dose metoclopramide, dexamethasone and diphenhydramine for cisplatin-induced emesis. An NCI Canada Clinical Trials Group phase III trial. Eur J Cancer. 1992;29A(1):33–6.
Gralla R, Lichinitser M, Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14(10):1570–7.
Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Cancer. 2003;98(11):2473–82.
Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10(2):115–24.
Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol. 2011;29(11):1495–501.
Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25(7):1328–33.
Aapro M, Karthaus M, Schwartzberg L, et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Support Care Cancer. 2017;25(4):1127–35.
Schwartzberg L, Roeland E, Andric Z, et al. Phase III safety study of intravenous NEPA: a novel fixed antiemetic combination of fosnetupitant and palonosetron in patients receiving highly emetogenic chemotherapy. Ann Oncol. 2018;29(7):1535–40.
Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16(9):1079–89.
Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. 2015;16(9):1071–8.
Grimison R, Mersiades A, Kirby A, et al. Oral TCH: CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomized, placebo-controlled, phase 2 crossover trial. Ann Oncol. 2020;31(11):1553–60.
Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9(5):188–95.
Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–42.
Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2020;21(2):242–9.
Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol. 2012;23(8):1986–92.
Jordan K, Chan A, Gralla RJ, et al. 2016 updated MASCC/ESMO consensus recommendations: emetic risk classification and evaluation of the emetogenicity of antineoplastic agents. Support Care Cancer. 2017;25(1):271–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
C. Kang is a salaried employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, consent to participate, consent for publication, availability of data and material, code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, C. Approach the prevention of chemotherapy-induced nausea and vomiting in older patients with care. Drugs Ther Perspect 38, 483–488 (2022). https://doi.org/10.1007/s40267-022-00952-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00952-4