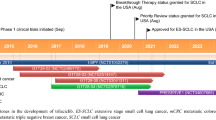
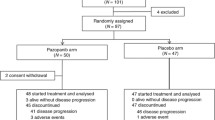
Abstract
Trilaciclib (COSELA™) is a transient inhibitor of cyclin-dependent kinases 4 and 6 (CDK 4/6) that is approved in the USA to decrease the incidence of chemotherapy-induced myelosuppression (CIM) when administered before a platinum/etoposide-containing regimen or topotecan-containing regimen in adults being treated for extensive-stage small cell lung cancer (ES-SCLC). It is the first approved therapy to provide myeloprotection, and when administered before chemotherapy, it is a valuable option for decreasing the incidence of CIM. In three clinical randomized trials, trilaciclib administration prior to standard-of-care chemotherapy for ES-SCLC reduced myelosuppression and the need for rescue interventions, improved patient-reported outcomes and the safety profile of the chemotherapy regimens, and had no impact on antitumor efficacy.
Plain Language Summary
As well as destroying cancer cells, chemotherapy also damages healthy, rapidly growing cells in the body, including blood-forming cells in the bone marrow (i.e. bone marrow suppression). This results in low levels of white blood cells (increasing the risk of infection), red blood cells (causing anemia) and platelets (increasing the risk of bleeding). Bone marrow suppression often occurs after treatment with the highly toxic chemotherapies, such as those used in the treatment of extensive-stage small cell lung cancer (ES-SCLC). Trilaciclib (COSELA™) is a drug that transiently protects the white and red blood cells and platelets from chemotherapy damage during treatment. When administered before chemotherapy, trilaciclib reduces the incidence of chemotherapy-induced bone marrow suppression. After treatment with trilaciclib and chemotherapy is completed, bone marrow cells resume growth and development. The need for supportive care treatments (including red cell and platelet transfusions and drugs that stimulate the production of white and red blood cells), chemotherapy dose reductions/delays and hospitalizations related to chemotherapy-induced bone marrow suppression and infection are reduced with trilaciclib treatment, and the overall safety profile of chemotherapy regimens is improved without affecting antitumour effects.
Similar content being viewed by others
References
He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(eaal3986):1–11.
Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606–18.
Parylo S, Vennepureddy A, Dhar V, et al. Role of cyclin-dependent kinase 4/6 inhibitors in the current and future eras of cancer treatment. J Oncol Pharm Pract. 2019;25(1):110–29.
Lyman GH, Kuderer NM, Aapro M. Improving outcomes of chemotherapy: established and novel options for myeloprotection in the COVID-19 era. Front Oncol. 2021;11:697908.
Ettl J. Management of adverse events due to cyclin-dependent kinase 4/6 inhibitors. Breast Care (Basel). 2019;14(2):86–92.
G1 Therapeutics Inc. COSELA™ (trilaciclib): US prescribing information. 2021. https://www.cosela.com/. Accessed 19 Nov 2021.
Roberts PJ, Kumarasamy V, Witkiewicz AK, et al. Chemotherapy and CDK4/6 inhibitors: unexpected bedfellows. Mol Cancer Ther. 2020;19(8):1575–88.
Weiss JM, Csoszi T, Maglakelidze M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30(10):1613–21.
Dhillon S. Trilaciclib: first approval. Drugs. 2021;81(7):867–74.
Li C, Hart L, Owonikoko TK, et al. Trilaciclib dose selection: an integrated pharmacokinetic and pharmacodynamic analysis of preclinical data and phase Ib/IIa studies in patients with extensive-stage small cell lung cancer. Cancer Chemother Pharmacol. 2021;87(5):689–700.
Hart LL, Ferrarotto R, Andric ZG, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: results from a randomized, double-blind, placebo-controlled phase II study. Adv Ther. 2021;38(1):350–65.
Daniel D, Kuchava V, Bondarenko I, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: a multicentre, randomised, double-blind, placebo-controlled phase II trial. Int J Cancer. 2020;148(10):2557–70.
Weiss J, Goldschmidt J, Andric Z, et al. Effects of trilaciclib on chemotherapy-induced myelosuppression and patient-reported outcomes in patients with extensive-stage small cell lung cancer: pooled results from three phase II randomized, double-blind, placebo-controlled studies. Clin Lung Cancer. 2021;22(5):449–60.
Ferrarotto R, Anderson I, Medgyasszay B, et al. Trilaciclib prior to chemotherapy reduces the usage of supportive care interventions for chemotherapy-induced myelosuppression in patients with small cell lung cancer: pooled analysis of three randomized phase 2 trials. Cancer Med. 2021;10(17):5748–56.
Hussein M, Maglakelidze M, Richards DA, et al. Myeloprotective effects of trilaciclib among patients with small cell lung cancer at increased risk of chemotherapy-induced myelosuppression: pooled results from three phase 2, randomized, double-blind, placebo-controlled studies. Cancer Manag Res. 2021;13:6207–18.
Dómine Gómez M, Csőszi T, Jaal J, et al. Exploratory composite endpoint demonstrates benefit of trilaciclib across multiple clinically meaningful components of myeloprotection in patients with small cell lung cancer. Int J Cancer. 2021;149(7):1463–72.
Tariq S, Kim SY, Monteiro de Oliveira Novaes J, et al. Update 2021: management of small cell lung cancer. Lung. 2021;199(6):579–87.
National Comprehensive Cancer Network. Small cell lung cancer (version 1.2022). 2021. https://www.nccn.org. Accessed 8 Oct 2021.
National Comprehensive Cancer Network. Hematopoietic growth factors (version 4.2021). 2021. https://www.nccn.org. Accessed 8 Oct 2021.
Acknowledgements
The manuscript was reviewed by: P.A. Bunn Jr., Department of Medicine, Division of Medical Oncology, University of Colorado Denver, Denver, CO, USA; R Tripathi, Department of Molecular and Biomedical Pharmacology, University of Kentucky School of Medicine, Lexington, KT, USA; F Rustemi, Health Insurance Fund, Tirana, Albania. During the peer review process, G1 Therapeutics, the marketing authorization holder of trilaciclib, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
ES Kim, a contracted employee of Adis International Ltd/Springer Nature, and SJ Keam, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, E.S., Keam, S.J. Trilaciclib for the reduction of chemotherapy-induced myelosuppression in the management of extensive-stage small cell lung cancer: a profile of its use. Drugs Ther Perspect 38, 64–71 (2022). https://doi.org/10.1007/s40267-021-00889-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00889-0