Abstract
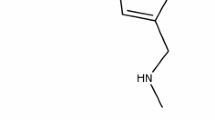
Zastaprazan (JAQBO®) is a next-generation potassium-competitive acid blocker being developed by Onconic Therapeutics, a subsidiary of Jeil Pharmaceutical, for the treatment of acid-related diseases. Zastaprazan binds directly to proton pumps in a competitive manner to reduce gastric acid secretion, allowing for a quick onset of action. On 24 April 2024, zastaprazan received approval in South Korea for the treatment of erosive gastroesophageal reflux disease (GERD). Zastaprazan is also undergoing phase III development for the treatment of gastric ulcer and for the prevention of non-steroidal anti-inflammatory drug (NSAID)-induced peptic ulcer. This article summarizes the milestones in the development of zastaprazan leading to this first approval for erosive GERD.
Similar content being viewed by others
References
Katzka DA, Pandolfino JE, Kahrilas PJ. Phenotypes of gastroesophageal reflux disease: where Rome, Lyon, and Montreal meet. Clin Gastroenterol Hepatol. 2020;18(4):767–76.
Armstrong D. Potassium-competitive acid blockers and gastroesophageal reflux disease. Gastroenterol Hepatol. 2023;19(5):295–8.
Onconic Therapeutics. Onconic Therapeutics receives MFDS approval for JAQBO, a new treatment for gastroesophageal reflux disease (GERD) [media release]. 25 Apr 2024. https://www.prnewswire.com/in/news-releases/onconic-therapeutics-receives-mfds-approval-for-jaqbo-a-new-treatment-for-gastroesophageal-reflux-disease-gerd-302127478.html.
Onconic Therapeutics. JAQBO 20 mg tablets (zastaprazan citrate): Korean prescribing information. 2024.
Livzon Pharmaceuticals. Voluntary announcement entering into the license agreement [media release]. 10 Mar 2023. http://www1.hkexnews.hk.
Ku JM, Cho JH, Kim K, et al. JP-1366: a novel and potent potassium-competitive acid blocker that is effective in the treatment of acid-related diseases. Pharmacol Res Perspect. 2023;11(3): e01090.
Hwang I, Ji SC, Oh J, et al. Randomised clinical trial: safety, tolerability, pharmacodynamics and pharmacokinetics of zastaprazan (JP-1366), a novel potassium-competitive acid blocker, in healthy subjects. Aliment Pharmacol Ther. 2023;57(7):763–72.
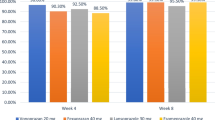
Oh J, Kim H, Cheung DY, et al. Randomised, double-blind, active-controlled phase 3 study to evaluate the efficacy and safety of zastaprazan compared with esomeprazole in erosive oesophagitis [zastaprazan erosive reflux oesophagitis-1 study (ZERO-1)] [abstract no. OP050]. United Eur Gastroenterol J. 2023;11(Suppl 8):54–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Hannah A. Blair is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics Approval, Consent to Participate, Consent to Publish, Availability of Data and Material, Code Availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blair, H.A. Zastaprazan: First Approval. Drugs (2024). https://doi.org/10.1007/s40265-024-02057-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02057-w