Abstract
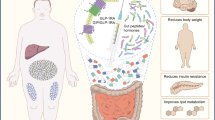
Tirzepatide (Mounjaro™) is a single molecule that combines dual agonism of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. Native GIP and GLP-1 are incretin hormones that stimulate insulin secretion and decrease glucagon secretion. GIP also plays a role in nutrient and energy metabolism, while GLP-1 also delays gastric emptying, supresses appetite and improves satiety. Eli Lilly is develo** tirzepatide for the treatment of type 2 diabetes mellitus (T2DM), obesity, cardiovascular disorders in T2DM, heart failure, non-alcoholic steatohepatitis, obstructive sleep apnoea and for reducing mortality/morbidity in obesity. In May 2022, tirzepatide received its first approval in the USA to improve glycaemic control in adults with T2DM, as an adjunct to diet and exercise. Tirzepatide is in phase III development for heart failure, obesity and cardiovascular disorders in T2DM, and in phase II development for non-alcoholic steatohepatitis. This article summarizes the milestones in the development of tirzepatide leading to this first approval for T2DM.
Similar content being viewed by others
References
Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14.
Eli Lilly and Company. Medicines in development: molecule & potential indication data as of April 28, 2022. https://www.lilly.com/discovery/clinical-development-pipeline. Accessed 23 June 2022.
Eli Lilly and Company. Lilly's tirzepatide delivered up to 22.5% weight loss in adults with obesity or overweight in SURMOUNT-1 [media release]. https://www.lilly.com/. Accessed 28 Apr 2022.
Fisman EZ, Tenenbaum A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect. Cardiovasc Diabetol. 2021;20(1):225.
Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 2021;12(1):143–57.
U.S. Food & Drug Administration. FDA approves novel, dual-targeted treatment for type 2 diabetes [media release]. https://www.fda.gov/. Accessed 13 May 2022.
Eli Lilly and Company. MOUNJAROTM (tirzepatide) injection, for subcutaneous use: US prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf. Accessed 23 Jun 2022.
Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(2):388–96.
Pirro V, Roth KD, Lin Y, et al. Effects of tirzepatide, a dual GIP and GLP-1 RA, on lipid and metabolite profiles in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2021;107(2):363–78.
Wilson JM, Lin Y, Luo MJ, et al. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: a post hoc analysis. Diabetes Obes Metab. 2022;24(1):148–53.
Wilson JM, Nikooienejad A, Robins DA, et al. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22(12):2451–9.
Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352–5.
Furihata K, Mimura H, Urva S, et al. A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes. Diabetes Obes Metab. 2022;24(2):239–46.
Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–93.
Frias JP, Nauck MA, Van J, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020;22(6):938–46.
Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–55.
Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15.
Ludvik B, Giorgino F, Jodar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–98.
Battelino T, Bergenstal RM, Rodríguez A, et al. Efficacy of once-weekly tirzepatide versus once-daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS-3 CGM): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):407–17.
Gastaldelli A, Cusi K, Fernández Landó L, et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022. https://doi.org/10.1016/s2213-8587(22)00070-5.
Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–24.
Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534–45.
US National Institutes of Health. ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT03861052. Accessed 23 June 2022.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2206038.
Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28(3):591–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Tirzepatide: First Approval. Drugs 82, 1213–1220 (2022). https://doi.org/10.1007/s40265-022-01746-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01746-8