Abstract
Introduction
Acute kidney injury is an expected adverse drug reaction listed in the European Union (EU) Summary of Product Characteristics (SmPC) for levetiracetam, one of the most widely used modern antiseizure medications (ASMs).
Objective
We conducted a voluntary post-authorization safety study to characterize the rate of acute renal failure (ARF) in patients exposed to levetiracetam versus other ASMs.
Methods
New users of ASMs without prior renal dysfunction were identified and followed for 30 days in the IBM® MarketScan® database (USA, January 2008–December 2017). ARF was defined as a diagnosis on inpatient or emergency department claims. We estimated adjusted incidence rates, incidence rate ratios (IRRs), and incidence rate differences (IRDs) of ARF in patients initiating levetiracetam versus other ASMs.
Results
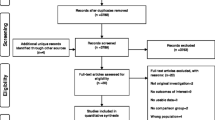
Overall, 110,336 patients were eligible for the monotherapy cohort and 96,215 were eligible for the polytherapy cohort. The overall crude rate of ARF following a new ASM was 6.0 and 6.5 per 10,000 patients for the ‘monotherapy’ and ‘polytherapy’ cohorts, respectively, in the first 30 days after the index date. In the monotherapy cohort, the IRR for ARF was 1.37 (95% confidence interval [CI] 0.80–2.34) and the corresponding IRD was 2.0 (95% CI − 1.12 to 5.12) additional ARFs per 10,000 patient-months. In the polytherapy cohort, the adjusted IRR for ARF was 0.94 (95% CI 0.51–1.74) and the corresponding IRD was − 0.42 cases per 10,000 patient-months (95% CI − 4.01 to 3.17).
Conclusions
The rate of ARFs in ASM new users was very low. In patients without prior ASMs, the estimated difference in risk of ARF associated with initiation of levetiracetam versus initiation of other ASMs was small, with 95% CIs compatible with small protective or harmful effects. In patients receiving polytherapy, the difference was compatible with the null and the 95% CI with small protective or harmful effects.



Similar content being viewed by others
References
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Beghi E, Giussani G, Nichols E, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):357–75.
Josephson CB, Engbers JDT, Jette N, Patten SB, Sajobi TT, Marshall D, et al. Prescription trends and psychiatric symptoms following first receipt of one of seven common antiepileptic drugs in general practice. Epilepsy Behav. 2018;84:49–55.
Powell G, Logan J, Kiri V, Borghs S. Trends in antiepileptic drug treatment and effectiveness in clinical practice in England from 2003 to 2016: a retrospective cohort study using electronic medical records. BMJ Open. 2019;9(12): e032551.
Thurman DJ, Faught E, Helmers S, Kim H, Kalilani L. New-onset lesional and nonlesional epilepsy in the US population: patient characteristics and patterns of antiepileptic drug use. Epilepsy Res. 2019;157: 106210.
Spengler DC, Montouris GD, Hohler AD. Levetiracetam as a possible contributor to acute kidney injury. Clin Ther. 2014;36(8):1303–6.
Choonara I, Star K. Levetiracetam and impaired renal function. WHO Pharm Newsl. 2016;2:18–23.
Vlasschaert MEO, Bejaimal SAD, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, et al. Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis. 2011;57(1):29–43.
Patel U, Hardy N, Smith D, Gurwitz J, Hsu C-Y, Parikh C, et al. Validation of acute kidney injury cases in the mini-sentinel distributed database. 2013. https://www.sentinelinitiative.org/sites/default/files/Drugs/Assessments/Mini-Sentinel_Validation-of-Acute-Kidney-Injury-Cases.pdf. Accessed 14 Apr 2022.
Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207.
Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6.
Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512–22.
Le HV, Poole C, Brookhart MA, Schoenbach VJ, Beach KJ, Layton JB, et al. Effects of aggregation of drug and diagnostic codes on the performance of the high-dimensional propensity score algorithm: an empirical example. BMC Med Res Methodol. 2013;13(1):142.
Agency for Healthcare Research and Quality. HCUP CCS-services and procedures. Healthcare Cost and Utilization Project (HCUP). October 2020. Rockville: Agency for Healthcare Research and Quality; 2020.
Agency for Healthcare Research and Quality. Tools archive for clinical classifications software refined. Healthcare Cost and Utilization Project (HCUP). March 2021. Rockville: Agency for Healthcare Research and Quality; 2021.
Yau K, Burneo JG, Jandoc R, McArthur E, Muanda FT, Parikh CR, et al. Population-based study of risk of AKI with levetiracetam. Clin J Am Soc Nephrol. 2019;14(1):17–26.
Faught E, Helmers S, Thurman D, Kim H, Kalilani L. Patient characteristics and treatment patterns in patients with newly diagnosed epilepsy: a US database analysis. Epilepsy Behav. 2018;85:37–44.
Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol. 2012;65(3):343-9.e2.
Greenland S, Lash T. Bias analysis. In: Rothman K, Greenland S, Lash T, editors. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 345–80.
Ryan PB, Schuemie MJ, Ramcharran D, Stang PE. Atypical antipsychotics and the risks of acute kidney injury and related outcomes among older adults: a replication analysis and an evaluation of adapted confounding control strategies. Drugs Aging. 2017;34(3):211–9.
Acknowledgments
Nada Boudiaf, David Friesen, and Kathrin Haeffs contributed to the validation of the analysis. The authors acknowledge Kathleen Richards, PhD (UCB Pharma, Smyrna, GA, USA) for publication coordination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Work on this manuscript was funded by UCB Pharma.
Conflicts of interest/competing interests
Lai San Hong was an independent contractor for UCB Pharma. Xabier Garcia de Albeniz and Susana Perez-Gutthann are employees of RTI-HS and were contracted to perform work on this study and manuscript, funded by UCB Pharma. Raphaelle Beau-Lejdstrom is a salaried employee of UCB Pharma and stockholder. Francois Bonfitto, Florin Floricel, Christian Loesch, and Isabelle Mottet are salaried UCB Pharma employees and stockholders. Linda Kalilani, Graham Luscombe, and Nadia Foskett were employees of UCB Pharma at the time this study was conducted. Johan Lorenzen has received fees for consulting and expert testimony relating to the subject matter discussed in this manuscript.
Availability of data and material
The dataset (IBM® MarketScan® Research Databases) generated and/or analyzed during the current study are not publicly available but are available from IBM® on reasonable request.
Code availability
Available from David Friesen (David.Friesen@ucb.com).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Authors’ contributions
RBL designed the study, contributed to the analysis, interpreted the data, and revised and approved the final version to be published. LSH contributed to the design of the study, analyzed the data, revised the manuscript, and approved the final version to be published. FF, JL, FB, LK, GL, CL, SPG, IM, and NF contributed to the design of the study and revised and approved the final version to be published. XGA contributed to the analysis and interpretation of the data, drafted the manuscript, and approved the final version to be published.
Rights and permissions
About this article
Cite this article
Beau-Lejdstrom, R., Hong, L.S., Garcia de Albeniz, X. et al. Incidence of Acute Renal Failure in Patients Using Levetiracetam Versus Other Antiseizure Medications: A Voluntary Post-Authorization Safety Study. Drug Saf 45, 781–790 (2022). https://doi.org/10.1007/s40264-022-01193-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01193-0