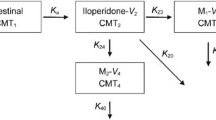
Abstract
Background and Objectives
Clozapine is the medication of choice for treatment-resistant schizophrenia. However, it has a complex metabolism and unexplained interindividual variability. The current work aims to develop a pharmacokinetic model of clozapine and norclozapine in non-smokers and assess the impact of demographic and genetic predictors.
Methods
Healthy volunteers were recruited in a population pharmacokinetic study. Blood samples were collected at 30 min and 1, 2, 3, 5 and 8 h following a single flat dose of clozapine (12.5 mg). The clozapine and norclozapine concentrations were measured via high-performance liquid chromatography–ultraviolet method. A semi-physiological pharmacokinetic model of clozapine and norclozapine was developed using nonlinear mixed-effects modeling. Clinical and genetic predictors were evaluated, including CYP1A2 (rs762551) and ABCB1 (rs2032582), using restriction fragment length polymorphism.
Results
A total of 270 samples were collected from 33 participants. The data were best described using a two-compartment model for clozapine and a two-compartment model for norclozapine with first-order absorption and elimination and pre-systemic metabolism. The estimated (relative standard error) clearance of clozapine and norclozapine were 27 L h-1 (31.5 %) and 19.6 L h-1 (30%), respectively. Clozapine clearance was lower in sub-Saharan Africans (n = 4) and higher in Caucasians (n = 9) than Asians (n = 20). Participants with CYP1A2 (rs762551) (n = 18) and ABCB1 (rs2032582) (n = 12) mutant alleles had lower clozapine clearance in the univariate analysis.
Conclusions
This is the first study to develop a semi-physiological pharmacokinetic model of clozapine and norclozapine accounting for the pre-systemic metabolism. Asians required lower doses of clozapine as compared with Caucasians, while clozapine pharmacokinetics in sub-Saharan Africans should be further investigated in larger trials.



Similar content being viewed by others
References
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216–29.
Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiat. 2019;76:1052–62.
Albitar O, Harun SN, Zainal H, Ibrahim B, Sheikh Ghadzi SM. Population pharmacokinetics of clozapine: a systematic review. Biomed Res Int. 2020;2020:1–10.
Schaber G, Stevens I, Gaertner HJ, Dietz K, Breyer-Pfaff U. Pharmacokinetics of clozapine and its metabolites in psychiatric patients: plasma protein binding and renal clearance. Br J Clin Pharmacol. 1998;46:453–9.
Albitar O, Ghadzi SMS, Harun SN, Ahmad SNA, Kjellsson MC. Pharmacometric modeling of drug adverse effects: an application of mixture models in schizophrenia spectrum disorder patients treated with clozapine. J Pharmacokinet Pharmacodyn. 2023;50:21–31.
Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44:195–235.
Yu H, Steeghs N, Kloth JSL, de Wit D, van Hasselt JGC, van Erp NP, et al. Integrated semi-physiological pharmacokinetic model for both sunitinib and its active metabolite SU12662. Br J Clin Pharmacol. 2015;79:809–19.
Albitar O, Murugaiyah V, Ibrahim B, Ahamed N, Sheikh Ghadzi SM. Clozapine and norclozapine monitoring in plasma following surfactant assisted dispersive liquid–liquid microextraction. Sep Sci Plus. 2022;5:55–64.
Beal SL, Shiener LB, Boeckman AJ. NONMEM users guides (1989–2008). Ellicott City: Icon Development Solutions; 2008.
Cheng YF, Lundberg T, Bondesson U, Lindström L, Gabrielsson J. Clinical pharmacokinetics of clozapine in chronic schizophrenic patients. Eur J Clin Pharmacol. 1988;34:445–9.
Jann MW, Griley SR, Gray EC, Chang WH. Pharmacokinetics and pharmacodynamics of clozapine. Clin. Pharmacokinet. Adis International Ltd; 1993. p. 161–76.
Fitton A, Heel RC. Clozapine: a review of its pharmacological properties, and therapeutic use in schizophrenia. Drugs. 1990;40:722–47.
Abduljalil K, Frank D, Gaedigk A, Klaassen T, Tomalik-Scharte D, Jetter A, et al. Assessment of activity levels for CYP2D6*1, CYP2D6*2, and CYP2D6*41 genes by population pharmacokinetics of dextromethorphan. Clin Pharmacol Ther. 2010;88:643–51.
Carlisle KM, Halliwell M, Read AE, Wells PNT. Estimation of total hepatic blood flow by duplex ultrasound. Gut. 1992;33:92–7.
Guitton C, Kinowski J-M, Gomeni R, Bressolle F. A kinetic model for simultaneous fit of clozapine and norclozapine concentrations in chronic schizophrenic patients during long-term treatment. Clin Drug Investig. 1998;16:35–43.
Chan Kwong AH-XP, Calvier EAM, Fabre D, Gattacceca F, Khier S. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: overview and guidance with a focus on the NONMEM PRIOR subroutine. J Pharmacokinet Pharmacodyn. 2020;47:431–46.
Keizer R, Karlsson M, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:1–9.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1998;58:51–64.
Hooker AC, Karlsson MO, Wilkins JJ, Jonsson EN. xpose4: Tools for nonlinear mixed-effect model building and diagnostics. R package version 4.6.1. (2014). http://xpose.sourceforge.net. Accessed 17 Feb 2020.
Keizer RJ, Zandvliet AS, Beijnen JH, Schellens JHM, Huitema ADR. Performance of methods for handling missing categorical covariate data in population pharmacokinetic analyses. AAPS J. 2012;14:601–11.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43:583–96.
Shang D-W, Li L-J, Wang X-P, Wen Y-G, Ren Y-P, Guo W, et al. Population pharmacokinetic/pharmacodynamic model of clozapine for characterizing the relationship between accumulated exposure and PANSS scores in patients with schizophrenia. Ther Drug Monit. 2014;36:378–86.
Choc MG, Lehr RG, Hsuan F, Honigfeld G, Smith HT, Borison R, et al. Multiple-dose pharmacokinetics of clozapine in patients. Pharm Res. 1987;04:402–5.
Jerling M, Merlé Y, Mentré F, Mallet A. Population pharmacokinetics of clozapine evaluated with the nonparametric maximum likelihood method. Br J Clin Pharmacol. 1997;44:447–53.
Dailly E, Urien S, Chanut E, Claudel B, Guerra N, Fernandez C, et al. Evidence from a population pharmacokinetics analysis for a major effect of CYP1A2 activity on inter- and intraindividual variations of clozapine clearance. Prog Neuro-Psychopharmacol Biol Psychiatry. 2002;26:699–703.
Doude van Troostwijk LJA, Koopmans RP, Vermeulen HD, Guchelaar H-J. CYP1A2 activity is an important determinant of clozapine dosage in schizophrenic patients. Eur J Pharm Sci. 2003;20:451–7.
Ng W, Uchida H, Ismail Z, Mamo DC, Rajji TK, Remington G, et al. Clozapine exposure and the impact of smoking and gender: a population pharmacokinetic study. Ther Drug Monit. 2009;31:360–6.
Ismail Z, Wessels AM, Uchida H, Ng W, Mamo DC, Rajji TK, et al. Age and sex impact clozapine plasma concentrations in inpatients and outpatients with schizophrenia. Am J Geriatr Psychiatry. 2012;20:53–60.
Li L, Shang D, Li W, Guo W, Wang X, Ren Y, et al. Population pharmacokinetics of clozapine and its primary metabolite norclozapine in Chinese patients with schizophrenia. Acta Pharmacol Sin. 2012;33:1409–16.
Li A-N, Dong F, He J-L, Shang D-W, Guo W, Li W-B, et al. The elimination rate after clozapine overdose in Chinese schizophrenia patients: a population pharmacokinetics model study. Pharmacopsychiatry. 2015;48:150–5.
Olmos I, Ibarra M, Vázquez M, Maldonado C, Fagiolino P, Giachetto G. Population pharmacokinetics of clozapine and norclozapine and switchability assessment between brands in uruguayan patients with schizophrenia. Biomed Res Int. 2019;2019:1–10.
Reeves S, Bertrand J, Obee SJ, Hunter S, Howard R, Flanagan RJ. A population pharmacokinetic model to guide clozapine dose selection, based on age, sex, ethnicity, body weight and smoking status. Br J Clin Pharmacol. 2024;90:135–45.
Qiu X-W, Fu P-X, Wang C-Y, Liu M, Zhou T-Y, Lu W. Population pharmacokinetics research of clozapine in Chinese schizophrenic patients. Yao Xue Xue Bao. 2009;44:785–92.
Özdemir V, Kalow W, Posner P, Collins EJ, Kennedy JL, Tang BK, et al. CYP1A2 activity as measured by a caffeine test predicts clozapine and active metabolite steady-state concentrationin patients with schizophrenia. J Clin Psychopharmacol. 2001;21:398–407.
Twesigomwe D, Drögemöller BI, Wright GEB, Adebamowo C, Agongo G, Boua PR, et al. Characterization of CYP2D6 pharmacogenetic variation in sub-Saharan African populations. Clin Pharmacol Ther. 2023;113:643–59.
Huddart R, Fohner AE, Whirl-Carrillo M, Wojcik GL, Gignoux CR, Popejoy AB, et al. Standardized biogeographic grou** system for annotating populations in pharmacogenetic research. Clin Pharmacol Ther. 2019;105:1256.
Ding J, Liu J, Zhang Y, **ng H, Zhang Y, Li L, et al. A retrospective study of clozapine and norclozapine concentration in patients with schizophrenia: data from the Therapeutic Drug Monitoring Service, 2019–2022. Asian J Psychiatr. 2024;91: 103865.
Flanagan RJ, Gee S, Belsey S, Couchman L, Lally J. Therapeutic monitoring of plasma clozapine and N-desmethylclozapine (norclozapine): practical considerations. BJPsych Adv. 2023;29:92–102.
Pardiñas AF, Kappel DB, Roberts M, Tipple F, Shitomi-Jones LM, King A, et al. Pharmacokinetics and pharmacogenomics of clozapine in an ancestrally diverse sample: a longitudinal analysis and genome-wide association study using UK clinical monitoring data. The Lancet Psychiatry. 2023;10:209–19.
De Leon J, Schoretsanitis G, Smith RL, Molden E, Solismaa A, Seppälä N, et al. An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry. Georg Thieme Verlag; 2022. p. 73–86.
Ruan CJ, Zang YN, Wang CY, Cheng YH, Sun C, Spina E, et al. Clozapine metabolism in East Asians and Caucasians: a pilot exploration of the prevalence of poor metabolizers and a systematic review. J Clin Psychopharmacol. 2019;39:135–44.
Singh R, Teo YY. “Asian” phenotype underestimates the genetic diversity of asia yet overstates its impact on variability in drug disposition and pharmacodynamics. Clin Pharmacol Ther. 2019;105:802–5.
Olsson E, Edman G, Bertilsson L, Hukic DS, Lavebratt C, Eriksson S V, et al. Genetic and clinical factors affecting plasma clozapine concentration. Prim Care Companion CNS Disord. 2015;17:1–16.
Jaquenoud Sirot E, Knezevic B, Morena GP, Harenberg S, Oneda B, Crettol S, et al. ABCB1 and cytochrome P450 polymorphisms. J Clin Psychopharmacol. 2009;29:319–26.
Kootstra-Ros JE, Smallegoor W, van der Weide J. The cytochrome P450 CYP1A2 genetic polymorphisms *1F and *1D do not affect clozapine clearance in a group of schizophrenic patients. Ann Clin Biochem. 2005;42:216–9.
Ortega-Vázquez A, Mayen-Lobo YG, Dávila-Ortiz de Montellano DJ, Tristán-López L, Aviña-Cervantes CL, Ríos C, et al. Alcohol intake potentiates clozapine adverse effects associated to CYP1A2*1C in patients with refractory psychosis. Drug Dev Res. 2021;82:685–94.
Sangüesa E, Cirujeda C, Concha J, Padilla PP, García CB, Ribate MP. Pharmacokinetic interactions between clozapine and valproic acid in patients with treatment-resistant schizophrenia: does UGT polymorphism affect these drug interactions? Chem Biol Interact. 2022;364: 110042.
Alarcan H, Cannet P, Camus V, Fond G, Zendjidjian X, Guilhaumou R, et al. Correlation between assessment of cytochrome P450 1A2 activity and enzyme activity scores, and their relation to clozapine exposure. Br J Clin Pharmacol. 2023;89:1665–71.
Islam F, Maciukiewicz M, Freeman N, Huang E, Tiwari A, Mulsant BH, et al. Contributions of cholinergic receptor muscarinic 1 and CYP1A2 gene variants on the effects of plasma ratio of clozapine/N-desmethylclozapine on working memory in schizophrenia. J Psychopharmacol. 2021;35:31–9.
Tian DD, Natesan S, White JR, Paine MF. Effects of common CYP1A2 genotypes and other key factors on intraindividual variation in the caffeine metabolic ratio: an exploratory analysis. Clin Transl Sci. 2019;12:39–46.
Piatkov I, Caetano D, Assur Y, Lau SL, Jones T, Boyages SC, et al. ABCB1 and ABCC1 single-nucleotide polymorphisms in patients treated with clozapine. Pharmgenom Pers Med. 2017;10:235–42.
Consoli G, Lastella M, Ciapparelli A, Dell’Osso MC, Ciofi L, Guidotti E, et al. ABCB1 polymorphisms are associated with clozapine plasma levels in psychotic patients. Pharmacogenomics. 2009;10:1267–76.
Krivoy A, Gaughran F, Weizman A, Breen G, MacCabe JH. Gene polymorphisms potentially related to the pharmacokinetics of clozapine. Int Clin Psychopharmacol. 2016;31:179–84.
Rajman I, Knapp L, Hanna I. Genetic diversity in drug transporters: impact in African populations. Clin Transl Sci. 2020;13:848.
Marazziti D, Mucci F, Avella MT, Palagini L, Simoncini M, Dell’Osso L. The increasing challenge of the possible impact of ethnicity on psychopharmacology. CNS Spectr. 2021;26:222–31.
Pardiñas AF, Nalmpanti M, Pocklington AJ, Legge SE, Medway C, King A, et al. Pharmacogenomic variants and drug interactions identified through the genetic analysis of clozapine metabolism. Am J Psychiatry. 2019;176:477–86.
Lenk HÇ, Løvsletten Smith R, O’Connell KS, Jukić MM, Kringen MK, Andreassen OA, et al. Impact of NFIB and CYP1A variants on clozapine serum concentration—a retrospective naturalistic cohort study on 526 patients with known smoking habits. Clin Transl Sci. 2023;16:62–72.
Berneri M, Jha U, O’Halloran S, Salman S, Wickramasinghe S, Kendrick K, et al. Validation of population pharmacokinetic models for clozapine dosage prediction. Ther Drug Monit. 2024;46:217–26.
Albitar O, Harun SN, Ahmad SNA, Sheikh Ghadzi SM. A repeated time-to-positive symptoms improvement among malaysian patients with schizophrenia spectrum disorders treated with clozapine. Pharmaceutics. 2021;13:1121.
Acknowledgments
This work has been supported by the Bridging Grant from Universiti Sains Malaysia (grant no. 304.PFARMASI.6316196). The authors acknowledge the USM Pusat Sejahtera staff for assisting in the clinical study and data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Bridging Grant, Universiti Sains Malaysia (Grant no. 304.PFARMASI.6316196).
Conflict of Interest:
The authors declare that they have no conflict of interest.
Ethics Approval
This study was approved by the Human Research Ethics Committee USM, Division of Research and Innovation (R&I), USM Health Campus, Malaysia (USM/JEPeM/20090488).
Consent to Participate
All participants provided written informed consent before enrollment into the study.
Consent for Publication
Not applicable.
Availability of Data and Material:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability:
The model structure and parameters were estimated using NONMEM 7.4.2 and the model code is provided in the supplementary.
Author Contributions
All authors have read and approved the final submitted manuscript, and agree to be accountable for the work. O.A. and S.M.S.G. designed the study. O.A. collected and analysed the data and drafted the manuscript. S.M.S.G. and S.N.H. supported the data analysis and finalised the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albitar, O., Harun, S.N. & Sheikh Ghadzi, S.M. Semi-physiological Pharmacokinetic Model of Clozapine and Norclozapine in Healthy, Non-smoking Volunteers: The Impact of Race and Genetics. CNS Drugs 38, 571–581 (2024). https://doi.org/10.1007/s40263-024-01092-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-024-01092-1