Abstract
Parkinson’s disease is the second most prevalent neurodegenerative disease and contributes significantly to morbidity globally. Currently, no disease-modifying therapies exist to combat this disorder. Insights from the molecular and cellular pathobiology of the disease seems to indicate promising therapeutic targets. The parkin protein has been extensively studied for its role in autosomal recessive Parkinson’s disease and, more recently, its role in sporadic Parkinson’s disease. Parkin is an E3 ubiquitin ligase that plays a prominent role in mitochondrial quality control, mitochondrial-dependent cell death pathways, and other diverse functions. Understanding the numerous roles of parkin has introduced many new possibilities for therapeutic modalities in treating both autosomal recessive Parkinson’s disease and sporadic Parkinson’s disease. In this article, we review parkin biology with an emphasis on mitochondrial-related functions and propose novel, potentially disease-modifying therapeutic approaches for treating this debilitating condition.


Similar content being viewed by others
References
Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis. 2020;6:15.
Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, et al.; Parkinson’s Foundation P4 Group. et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21.
Lang AE, Lozano AM. Parkinson’s disease. N Engl J Med. 1998;339:1130–43.
Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–54.
Giguère N, Burke Nanni S, Trudeau L-E. On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol. 2018;9:455.
Pankratz N, Foroud T. Genetics of Parkinson disease. Genet Med. 2007;9:801–11.
Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19:170–8.
Hernandez DG, Reed X, Singleton AB. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem. 2016;139:59–74.
von Coelln R, Dawson VL, Dawson TM. Parkin-associated Parkinson’s disease. Cell Tissue Res. 2004;318:175–84.
Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, Gasser T, et al. Association between early-onset Parkinson’s disease and mutations in the Parkin gene. N Engl J Med. 2000;342:1560–7.
Sun Y, Yu H, Zhou X, **ong W, Luo S, Chen C, et al. Disease progression in patients with Parkin related Parkinson’s disease in a longitudinal cohort. Mov Disord. 2020;mds.28349.
Kam T-I, Mao X, Park H, Chou S-C, Karuppagounder SS, Umanah GE, et al. Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease. Science. 2018;362:eaat8407.
Ham S, Yun SP, Kim H, Kim D, Seo BA, Kim H, et al. Amyloid-like oligomerization of AIMP2 contributes to α-synuclein interaction and Lewy-like inclusion. Sci Transl Med. 2020;12:eaax0091.
Chung KKK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, et al. Parkin ubiquitinates the α-synuclein–interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–50.
Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E, et al. Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann Neurol. 2004;55:439–42.
Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H. Synphilin-1 is present in Lewy bodies in Parkinson’s disease. Ann Neurol. 2000;47:521–3.
Shimura H, Hattori N, Kubo S, Yoshikawa M, Kitada T, Matsumine H, et al. Immunohistochemical and subcellular localization of parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Ann Neurol. 1999;45:668–72.
Hayashi S, Wakabayashi K, Ishikawa A, Nagai H, Saito M, Maruyama M, et al. An autopsy case of autosomal-recessive juvenile parkinsonism with a homozygous exon 4 deletion in the parkin gene. Mov Disord. 2000;15:884–8.
Takahashi H, Ohama E, Suzuki S, Horikawa Y, Ishikawa A, Morita T, et al. Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology. 1994;44:437–41.
Farrer M, Chan P, Chen R, Tan L, Lincoln S, Hernandez D, et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol. 2001;50:293–300.
Espay AJ, Brundin P, Lang AE. Precision medicine for disease modification in Parkinson disease. Nat Rev Neurol. 2017;13:119–26.
Antonini A, Moro E, Godeiro C, Reichmann H. Medical and surgical management of advanced Parkinson’s disease: management of advanced Parkinson’s disease. Mov Disord. 2018;33:900–8.
Lang AE, Espay AJ. Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations: disease modification in PD. Mov Disord. 2018;33:660–77.
Lozano AM, Dostrovsky J, Chen R, Ashby P. Deep brain stimulation for Parkinson’s disease: disrupting the disruption. Lancet Neurol. 2002;1:225–31.
Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–57.
Amadoro G, Corsetti V, Florenzano F, Atlante A, Bobba A, Nicolin V, et al. Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: the PINK-parkin pathway. Front Aging Neurosci. 2014;6:18.
Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18:101–13.
Misgeld T, Schwarz TL. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron. 2017;96:651–66.
Pacelli C, Giguère N, Bourque M-J, Lévesque M, Slack RS, Trudeau L-É. Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol. 2015;25:2349–60.
Giguère N, Pacelli C, Saumure C, Bourque M-J, Matheoud D, Levesque D, et al. Comparative analysis of Parkinson’s disease-associated genes in mice reveals altered survival and bioenergetics of parkin-deficient dopamine neurons. J Biol Chem. 2018;293:9580–93.
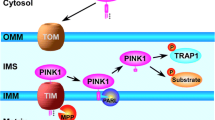
Pickrell AM, Youle RJ. The Roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–73.
Rüb C, Wilkening A, Voos W. Mitochondrial quality control by the Pink1/parkin system. Cell Tissue Res. 2017;367:111–23.
Voigt A, Berlemann LA, Winklhofer KF. The mitochondrial kinase PINK1: functions beyond mitophagy. J Neurochem. 2016;139:232–9.
Rasool S, Soya N, Truong L, Croteau N, Lukacs GL, Trempe J-F. PINK1 autophosphorylation is required for ubiquitin recognition. EMBO Rep. 2018;19: e44981.
Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–6.
Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, et al. PINK1 phosphorylates ubiquitin to activate parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205:143–53.
Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, et al. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460:127–39.
Wong YC, Holzbaur ELF. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA. 2014;111:E4439–48.
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–14.
Ge P, Dawson VL, Dawson TM. PINK1 and parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol Neurodegener. 2020;15:20.
Sung H, Tandarich LC, Nguyen K, Hollenbeck PJ. Compartmentalized regulation of parkin-mediated mitochondrial quality control in the Drosophila nervous system in vivo. J Neurosci. 2016;36:7375–91.
Pickrell AM, Huang C-H, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, et al. Endogenous parkin preserves dopaminergic substantia nigral neurons following mitochondrial DNA mutagenic stress. Neuron. 2015;87:371–81.
Van Laar VS, Arnold B, Cassady SJ, Chu CT, Burton EA, Berman SB. Bioenergetics of neurons inhibit the translocation response of parkin following rapid mitochondrial depolarization. Hum Mol Genet. 2011;20:927–40.
Cai Q, Zakaria HM, Simone A, Sheng Z-H. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol. 2012;22:545–52.
Lee JJ, Sanchez-Martinez A, Zarate AM, Benincá C, Mayor U, Clague MJ, et al. Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J Cell Biol. 2018;217:1613–22.
McWilliams TG, Prescott AR, Montava-Garriga L, Ball G, Singh F, Barini E, et al. Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metab. 2018;27:439-49.e5.
Kim YY, Um J, Yoon J, Kim H, Lee D, Lee YJ, et al. Assessment of mitophagy in mt-Keima Drosophila revealed an essential role of the PINK1-parkin pathway in mitophagy induction in vivo. FASEB J. 2019;33:9742–51.
Cornelissen T, Vilain S, Vints K, Gounko N, Verstreken P, Vandenberghe W. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. Elife. 2018;7:e35878.
Devireddy S, Liu A, Lampe T, Hollenbeck PJ. The organization of Mitochondrial quality control and life cycle in the nervous system in vivo in the absence of PINK1. J Neurosci. 2015;35:9391–401.
Stevens DA, Lee Y, Kang HC, Lee BD, Lee Y-I, Bower A, et al. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc Natl Acad Sci USA. 2015;112:11696–701.
Schwartzentruber A, Boschian C, Lopes FM, Myszczynska MA, New EJ, Beyrath J, et al. Oxidative switch drives mitophagy defects in dopaminergic parkin mutant patient neurons. Sci Rep. 2020;10:15485.
Bus C, Zizmare L, Feldkaemper M, Geisler S, Zarani M, Schaedler A, et al. Human dopaminergic neurons lacking PINK1 exhibit disrupted dopamine metabolism related to vitamin B6 co-factors. iScience. 2020;23:101797.
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–61.
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila PINK1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–6.
Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–5.
Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates Mitofusin. Proc Natl Acad Sci. 2010;107:5018–23.
Yu W, Sun Y, Guo S, Lu B. The PINK1/parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum Mol Genet. 2011;20:3227–40.
Glauser L, Sonnay S, Stafa K, Moore DJ. Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1: parkin promotes the ubiquitination of mitofusin 1. J Neurochem. 2011;118:636–45.
Chung SY, Kishinevsky S, Mazzulli JR, Graziotto J, Mrejeru A, Mosharov EV, et al. Parkin and PINK1 patient iPSC-derived midbrain dopamine neurons exhibit mitochondrial dysfunction and α-synuclein accumulation. Stem Cell Rep. 2016;7:664–77.
Shaltouki A, Sivapatham R, Pei Y, Gerencser AA, Momčilović O, Rao MS, et al. Mitochondrial alterations by parkin in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Rep. 2015;4:847–59.
Panicker N, Ge P, Dawson VL, Dawson TM. The cell biology of Parkinson’s disease. J Cell Biol. 2021;220: e202012095.
Manzella N, Santin Y, Maggiorani D, Martini H, Douin-Echinard V, Passos JF, et al. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell. 2018;17: e12811.
Yu W, Gao B, Li N, Wang J, Qiu C, Zhang G, et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: role of Foxo3A-parkin-mediated mitophagy. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1973–83.
Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, et al. Cytosolic p53 inhibits parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013;4:2308.
Song M, Gong G, Burelle Y, Gustafsson ÅB, Kitsis RN, Matkovich SJ, et al. Interdependence of parkin-mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ Res. 2015;117:346–51.
Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69.
Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease: a double-edged sword. Neuron. 2002;35:419–32.
Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–97.
Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:S210–2.
Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson’s disease. J Park Dis. 2013;3:493–514.
Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2: a009381.
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–47.
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53.
Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee J-K, Tansey MG. Lipopolysaccharide and tumor necrosis factor regulate parkin expression via nuclear factor-kappa B. PLoS ONE. 2011;6: e23660.
Mouton-Liger F, Rosazza T, Sepulveda-Diaz J, Ieang A, Hassoun S-M, Claire E, et al. Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia. 2018;66:1736–51.
Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, et al. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci USA. 2014;111:15514–9.
Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910.
Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–35.
von Coelln R, Thomas B, Andrabi SA, Lim KL, Savitt JM, Saffary R, et al. Inclusion body formation and neurodegeneration are parkin independent in a mouse model of alpha-synucleinopathy. J Neurosci. 2006;26:3685–96.
Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–34.
Sugiura A, McLelland G, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–56.
Abuaita BH, Schultz TL, O’Riordan MX. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized Staphylococcus aureus. Cell Host Microbe. 2018;24:625-36.e5.
McLelland G-L, Lee SA, McBride HM, Fon EA. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J Cell Biol. 2016;214:275–91.
Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS ONE. 2012;7: e52830.
Soubannier V, McLelland G-L, Zunino R, Braschi E, Rippstein P, Fon EA, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22:135–41.
Cadete VJJ, Deschênes S, Cuillerier A, Brisebois F, Sugiura A, Vincent A, et al. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system: cardiac mitochondrial-derived vesicle formation. J Physiol. 2016;594:5343–62.
Williams ET, Glauser L, Tsika E, Jiang H, Islam S, Moore DJ. Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum Mol Genet. 2018;27:3189–205.
Yun SP, Kim H, Ham S, Kwon S-H, Lee GH, Shin J-H, et al. VPS35 regulates parkin substrate AIMP2 toxicity by facilitating lysosomal clearance of AIMP2. Cell Death Dis. 2017;8:e2741–e2741.
Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, et al. Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell. 2016;166:314–27.
Cebrián C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633.
Martin HL, Santoro M, Mustafa S, Riedel G, Forrester JV, Teismann P. Evidence for a role of adaptive immune response in the disease pathogenesis of the MPTP mouse model of Parkinson’s disease: MHC II Ablation Attenuates MPTP Toxicity. Glia. 2016;64:386–95.
Ryan TA, Phillips EO, Collier CL, Robinson JBA, Routledge D, Wood RE, et al. Tollip coordinates parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020;39:e102539.
Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–62.
Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, et al. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11:1386.
Haenseler W, Zambon F, Lee H, Vowles J, Rinaldi F, Duggal G, et al. Excess α-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci Rep. 2017;7:9003.
Tremblay M-E, Cookson MR, Civiero L. Glial phagocytic clearance in Parkinson’s disease. Mol Neurodegener. 2019;14:16.
Ali S, Vollaard AM, Widjaja S, Surjadi C, van de Vosse E, van Dissel JT. PARK2/PACRG polymorphisms and susceptibility to typhoid and paratyphoid fever. Clin Exp Immunol. 2006;144:425–31.
Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–6.
Mira MT, Alcaïs A, Nguyen VT, Moraes MO, Di Flumeri C, Vu HT, et al. Susceptibility to leprosy is associated with PARK2 and PACRG. Nature. 2004;427:636–40.
Vergara D, Ferraro MM, Cascione M, del Mercato LL, Leporatti S, Ferretta A, et al. Cytoskeletal alterations and biomechanical properties of parkin-mutant human primary fibroblasts. Cell Biochem Biophys. 2015;71:1395–404.
Lim MK, Kawamura T, Ohsawa Y, Ohtsubo M, Asakawa S, Takayanagi A, et al. Parkin interacts with LIM Kinase 1 and reduces its cofilin-phosphorylation activity via ubiquitination. Exp Cell Res. 2007;313:2858–74.
Yang F, Jiang Q, Zhao J, Ren Y, Sutton MD, Feng J. Parkin stabilizes microtubules through strong binding mediated by three independent domains. J Biol Chem. 2005;280:17154–62.
Guttenplan KA, Weigel MK, Prakash P, Wijewardhane PR, Hasel P, Rufen-Blanchette U, et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature. 2021;599:102–7.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7.
Kano M, Takanashi M, Oyama G, Yoritaka A, Hatano T, Shiba-Fukushima K, et al. Reduced astrocytic reactivity in human brains and midbrain organoids with PRKN mutations. NPJ Parkinsons Dis. 2020;6:33.
Yun SP, Kam T-I, Panicker N, Kim S, Oh Y, Park J-S, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–8.
Sonninen T-M, Hämäläinen RH, Koskuvi M, Oksanen M, Shakirzyanova A, Wojciechowski S, et al. Metabolic alterations in Parkinson’s disease astrocytes. Sci Rep. 2020;10:14474.
Wu J, Dobbs N, Yang K, Yan N. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. 2020;53:115-126.e5.
Yamashiro LH, Wilson SC, Morrison HM, Karalis V, Chung J-YJ, Chen KJ, et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat Commun. 2020;11:3382.
Nassour J, Radford R, Correia A, Fusté JM, Schoell B, Jauch A, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–63.
Margolis SR, Wilson SC, Vance RE. Evolutionary origins of cGAS-STING signaling. Trends Immunol. 2017;38:733–43.
Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J Immunol. 2018;200:2748–56.
Glück S, Guey B, Gulen MF, Wolter K, Kang T-W, Schmacke NA, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol. 2017;19:1061–70.
Gulen MF, Koch U, Haag SM, Schuler F, Apetoh L, Villunger A, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8:427.
San Luciano M, Tanner CM, Meng C, Marras C, Goldman SM, Lang AE, et al. Nonsteroidal anti-inflammatory use and lrrk2 Parkinson’s disease penetrance. Mov Disord. 2020;35:1755–64.
Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010;74:995–1002.
Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836–42.
Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76:863–9.
Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S-S890.
Finck BN. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22.
Hu Q, Wang G. Mitochondrial dysfunction in Parkinson’s disease. Transl Neurodegener. 2016;5:14.
Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ. Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem Sci. 2021;46:329–43.
Clark J, Reddy S, Zheng K, Betensky RA, Simon DK. Association of PGC-1alphapolymorphisms with age of onset and risk of Parkinson’s disease. BMC Med Genet. 2011;12:69.
Su X, Chu Y, Kordower JH, Li B, Cao H, Huang L, et al. PGC-1α promoter methylation in Parkinson’s disease. PLoS ONE. 2015;10: e0134087.
Pacelli C, De Rasmo D, Signorile A, Grattagliano I, di Tullio G, D’Orazio A, et al. Mitochondrial defect and PGC-1α dysfunction in parkin-associated familial Parkinson’s disease. Biochim Biophys Acta. 2011;1812:1041–53.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408.
Shin J-H, Ko HS, Kang H, Lee Y, Lee Y-I, Pletinkova O, et al. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702.
Lee Y, Stevens DA, Kang S-U, Jiang H, Lee Y-I, Ko HS, et al. PINK1 primes parkin-mediated ubiquitination of PARIS in dopaminergic neuronal survival. Cell Rep. 2017;18:918–32.
Pirooznia SK, Yuan C, Khan MR, Karuppagounder SS, Wang L, **ong Y, et al. PARIS induced defects in mitochondrial biogenesis drive dopamine neuron loss under conditions of parkin or PINK1 deficiency. Mol Neurodegener. 2020;15:17.
Siddiqui A, Bhaumik D, Chinta SJ, Rane A, Rajagopalan S, Lieu CA, et al. Mitochondrial quality control via the PGC1α-TFEB signaling pathway is compromised by parkin Q311X mutation but independently restored by rapamycin. J Neurosci. 2015;35:12833–44.
Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RAV, et al. PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med. 2012;4:142ra97.
Martini-Stoica H, Xu Y, Ballabio A, Zheng H. The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci. 2016;39:221–34.
Brahmachari S, Lee S, Kim S, Yuan C, Karuppagounder SS, Ge P, et al. Parkin interacting substrate zinc finger protein 746 is a pathological mediator in Parkinson’s disease. Brain. 2019;142:2380–401.
Kim H, Shin J-Y, Jo A, Kim JH, Park S, Choi J-Y, et al. Parkin interacting substrate phosphorylation by c-Abl drives dopaminergic neurodegeneration. Brain. 2021;144:3674–91.
Panicker N, Kam T-I, Wang H, Neifert S, Chou S-C, Kumar M, et al. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson’s disease. Neuron. 2022;110:2422-37.e9.
Kumar M, Acevedo-Cintrón J, Jhaldiyal A, Wang H, Andrabi SA, Eacker S, et al. Defects in mitochondrial biogenesis drive mitochondrial alterations in parkin-deficient human dopamine neurons. Stem Cell Rep. 2020;15:629–45.
Nishida T, Yamada Y. RNF4-mediated SUMO-targeted ubiquitination relieves PARIS/ZNF746-mediated transcriptional repression. Biochem Biophys Res Commun. 2020;526:110–6.
Jo A, Lee Y, Kam T-I, Kang S-U, Neifert S, Karuppagounder SS, et al. PARIS farnesylation prevents neurodegeneration in models of Parkinson’s disease. Sci Transl Med. 2021;13:eaax8891.
Clark J, Silvaggi JM, Kiselak T, Zheng K, Clore EL, Dai Y, et al. Pgc-1α overexpression downregulates Pitx3 and increases susceptibility to MPTP toxicity associated with decreased Bdnf. PLoS ONE. 2012;7: e48925.
Ciron C, Lengacher S, Dusonchet J, Aebischer P, Schneider BL. Sustained expression of PGC-1 in the rat nigrostriatal system selectively impairs dopaminergic function. Hum Mol Genet. 2012;21:1861–76.
Johnson BN, Berger AK, Cortese GP, Lavoie MJ. The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc Natl Acad Sci USA. 2012;109:6283–8.
Bernardini JP, Brouwer JM, Tan IK, Sandow JJ, Huang S, Stafford CA, et al. Parkin inhibits BAK and BAX apoptotic function by distinct mechanisms during mitophagy. EMBO J. 2019;38: e99916.
Ham SJ, Lee D, Yoo H, Jun K, Shin H, Chung J. Decision between mitophagy and apoptosis by parkin via VDAC1 ubiquitination. Proc Natl Acad Sci. 2020;117:4281–91.
McLelland G-L, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–95.
Carroll RG, Hollville E, Martin SJ. Parkin sensitizes toward apoptosis induced by mitochondrial depolarization through promoting degradation of Mcl-1. Cell Rep. 2014;9:1538–53.
Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171:2000–16.
Lee Y, Karuppagounder SS, Shin J-H, Lee Y-I, Ko HS, Swing D, et al. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci. 2013;16:1392–400.
Trempe J-F, Sauve V, Grenier K, Seirafi M, Tang MY, Menade M, et al. Structure of parkin reveals mechanisms for ubiquitin ligase activation. Science. 2013;340:1451–5.
Seirafi M, Kozlov G, Gehring K. Parkin structure and function. FEBS J. 2015;282:2076–88.
Tang MY, Vranas M, Krahn AI, Pundlik S, Trempe J-F, Fon EA. Structure-guided mutagenesis reveals a hierarchical mechanism of parkin activation. Nat Commun. 2017;8:14697.
Sauvé V, Sung G, Soya N, Kozlov G, Blaimschein N, Miotto LS, et al. Mechanism of parkin activation by phosphorylation. Nat Struct Mol Biol. 2018;25:623–30.
Aguirre JD, Dunkerley KM, Mercier P, Shaw GS. Structure of phosphorylated UBL domain and insights into PINK1-orchestrated parkin activation. Proc Natl Acad Sci. 2017;114:298–303.
Miller S, Muqit MMK. Therapeutic approaches to enhance PINK1/parkin mediated mitophagy for the treatment of Parkinson’s disease. Neurosci Lett. 2019;705:7–13.
Wauer T, Komander D. Structure of the human Parkin ligase domain in an autoinhibited state. EMBO J. 2013;32:2099–112.
Panicker N, Dawson VL, Dawson TM. Activation mechanisms of the E3 ubiquitin ligase parkin. Biochem J. 2017;474:3075–86.
Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, et al. A neo-substrate that amplifies catalytic activity of Parkinson’s-disease-related kinase PINK1. Cell. 2013;154:737–47.
Osgerby L, Lai Y-C, Thornton PJ, Amalfitano J, Le Duff CS, Jabeen I, et al. Kinetin riboside and its ProTides Aativate the Parkinson’s Disease Associated PTEN-Induced Putative Kinase 1 (PINK1) independent of mitochondrial depolarization. J Med Chem. 2017;60:3518–24.
Imam SZ, Zhou Q, Yamamoto A, Valente AJ, Ali SF, Bains M, et al. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J Neurosci. 2011;31:157–63.
Ko HS, Lee Y, Shin J-H, Karuppagounder SS, Gadad BS, Koleske AJ, et al. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci USA. 2010;107:16691–6.
Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, Ko HS. The c-Abl inhibitor, Nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Sci Rep. 2015;4:4874.
Lonskaya I, Desforges NM, Hebron ML, Moussa CE-H. Ubiquitination increases parkin activity to promote autophagic α-synuclein clearance. PLoS ONE. 2013;8:e83914.
Lonskaya I, Hebron ML, Desforges NM, Franjie A, Moussa CE-H. Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol Med. 2013;5:1247–62.
Simuni T, Fiske B, Merchant K, Coffey CS, Klingner E, Caspell-Garcia C, et al. Efficacy of nilotinib in patients with moderately advanced Parkinson disease: a randomized clinical trial. JAMA Neurol. 2021;78:312.
Pagan Fernando L, Hebron ML, Wilmarth B, Torres-Yaghi Y, Lawler A, Mundel EE, et al. Nilotinib effects on safety, tolerability, and potential biomarkers in Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2020;77:309.
Fowler AJ, Hebron M, Missner AA, Wang R, Gao X, Kurd-Misto BT, et al. Multikinase Abl/DDR/Src inhibition produces optimal effects for tyrosine kinase inhibition in neurodegeneration. Drugs RD. 2019;19:149–66.
Pagan FL, Wilmarth B, Torres-Yaghi Y, Hebron ML, Mulki S, Ferrante D, et al. Long-term safety and clinical effects of nilotinib in Parkinson’s disease. Mov Disord. 2021;36:740–9.
Fowler AJ, Ahn J, Hebron M, Chiu T, Ayoub R, Mulki S, et al. CSF microRNAs reveal impairment of angiogenesis and autophagy in Parkinson disease. Neurol Genet. 2021;7: e633.
Imam SZ, Trickler W, Kimura S, Binienda ZK, Paule MG, Slikker W, et al. Neuroprotective efficacy of a new brain-penetrating C-Abl inhibitor in a murine Parkinson’s disease model. PLoS ONE. 2013;8: e65129.
Ko HS, Lee Y, Shin J-H, Karuppagounder SS, Gadad BS, Koleske AJ, et al. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci. 2010;107:16691–6.
Durcan TM, Tang MY, Pérusse JR, Dashti EA, Aguileta MA, McLelland G, et al. usp 8 regulates mitophagy by removing k 6-linked ubiquitin conjugates from parkin. EMBO J. 2014;33:2473–91.
Durcan TM, Fon EA. Mutant ataxin-3 promotes the autophagic degradation of parkin. Autophagy. 2011;7:233–4.
Durcan TM, Kontogiannea M, Bedard N, Wing SS, Fon EA. Ataxin-3 deubiquitination is coupled to parkin ubiquitination via E2 ubiquitin-conjugating enzyme*. J Biol Chem. 2012;287:531–41.
Chung KKK. S-Nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304:1328–31.
Oh C-K, Sultan A, Platzer J, Dolatabadi N, Soldner F, McClatchy DB, et al. S-Nitrosylation of PINK1 attenuates PINK1/parkin-dependent mitophagy in hiPSC-based Parkinson’s Ddsease models. Cell Rep. 2017;21:2171–82.
Yao D, Gu Z, Nakamura T, Shi Z-Q, Ma Y, Gaston B, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci. 2004;101:10810–4.
Ozawa K, Tsumoto H, Miura Y, Yamaguchi J, Iguchi-Ariga SMM, Sakuma T, et al. DJ-1 is indispensable for the S-nitrosylation of parkin, which maintains function of mitochondria. Sci Rep. 2020;10:4377.
Wilkaniec A, Lenkiewicz AM, Czapski GA, Jęśko HM, Hilgier W, Brodzik R, et al. Extracellular alpha-synuclein oligomers induce parkin S-nitrosylation: relevance to sporadic Parkinson’s disease etiopathology. Mol Neurobiol. 2019;56:125–40.
Sunico CR, Nakamura T, Rockenstein E, Mante M, Adame A, Chan S, et al. S-Nitrosylation of parkin as a novel regulator of p53-mediated neuronal cell death in sporadic Parkinson’s disease. Mol Neurodegener. 2013;8:29.
Um JW, Chung KC. Functional modulation of parkin through physical interaction with SUMO-1. J Neurosci Res. 2006;84:1543–54.
Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626.
Choo YS, Vogler G, Wang D, Kalvakuri S, Iliuk A, Tao WA, et al. Regulation of parkin and PINK1 by neddylation. Hum Mol Genet. 2012;21:2514–23.
Um JW, Han KA, Im E, Oh Y, Lee K, Chung KC. Neddylation positively regulates the ubiquitin E3 ligase activity of parkin. J Neurosci Res. 2012;90:1030–42.
Moskal N, Riccio V, Bashkurov M, Taddese R, Datti A, Lewis PN, et al. ROCK inhibitors upregulate the neuroprotective parkin-mediated mitophagy pathway. Nat Commun. 2020;11:88.
Saal K-A, Galter D, Roeber S, Bähr M, Tönges L, Lingor P. Altered expression of growth associated protein-43 and Rho kinase in human patients with Parkinson’s disease. Brain Pathol Zurich Switz. 2017;27:13–25.
Tonges L, Frank T, Tatenhorst L, Saal KA, Koch JC, Szego EM, et al. Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain. 2012;135:3355–70.
Koch JC, Tatenhorst L, Roser A-E, Saal K-A, Tönges L, Lingor P. ROCK inhibition in models of neurodegeneration and its potential for clinical translation. Pharmacol Ther. 2018;189:1–21.
Hasson SA, Kane LA, Yamano K, Huang C-H, Sliter DA, Buehler E, et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature. 2013;504:291–5.
Ivatt RM, Sanchez-Martinez A, Godena VK, Brown S, Ziviani E, Whitworth AJ. Genome-wide RNAi screen identifies the Parkinson disease GWAS risk locus SREBF1 as a regulator of mitophagy. Proc Natl Acad Sci U S A. 2014;111:8494–9.
Sekine S, Wang C, Sideris DP, Bunker E, Zhang Z, Youle RJ. Reciprocal roles of Tom7 and OMA1 during mitochondrial import and activation of PINK1. Mol Cell. 2019;73:1028-43.e5.
Liu X, Hebron M, Shi W, Lonskaya I, Moussa CE-H. Ubiquitin specific protease-13 independently regulates parkin ubiquitination and alpha-synuclein clearance in alpha-synucleinopathies. Hum Mol Genet. 2019;28:548–60.
Khandelwal PJ, Dumanis SB, Feng LR, Maguire-Zeiss K, Rebeck G, Lashuel HA, et al. Parkinson-related parkin reduces α-Synuclein phosphorylation in a gene transfer model. Mol Neurodegener. 2010;5:47.
Lonskaya I, Hebron ML, Algarzae NK, Desforges N, Moussa CE-H. Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson’s disease. Neuroscience. 2013;232:90–105.
Herman AM, Moussa CE-H. The ubiquitin ligase parkin modulates the execution of autophagy. Autophagy. 2011;7:919–21.
Khandelwal PJ, Herman AM, Hoe H-S, Rebeck GW, Moussa CE-H. Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Abeta in AD models. Hum Mol Genet. 2011;20:2091–102.
Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, et al. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510:370–5.
Tsefou E, Walker AS, Clark EH, Hicks AR, Luft C, Takeda K, et al. Investigation of USP30 inhibition to enhance parkin-mediated mitophagy: tools and approaches. Biochem J. 2021;478:4099–118.
Cornelissen T, Haddad D, Wauters F, Van Humbeeck C, Mandemakers W, Koentjoro B, et al. The deubiquitinase USP15 antagonizes parkin-mediated mitochondrial ubiquitination and mitophagy. Hum Mol Genet. 2014;23:5227–42.
Pirooznia SK, Wang H, Panicker N, Kumar M, Neifert S, Dar MA, et al. Deubiquitinase CYLD acts as a negative regulator of dopamine neuron survival in Parkinson’s disease. Sci Adv. 2022;8:eabh1824.
Dobson-Stone C, Hallupp M, Shahheydari H, Ragagnin AMG, Chatterton Z, Carew-Jones F, et al. CYLD is a causative gene for frontotemporal dementia-amyotrophic lateral sclerosis. Brain. 2020;143:783–99.
Dobson-Stone C, Luty AA, Thompson EM, Blumbergs P, Brooks WS, Short CL, et al. Frontotemporal dementia-amyotrophic lateral sclerosis syndrome locus on chromosome 16p12.1–q12.2: genetic, clinical and neuropathological analysis. Acta Neuropathol (Berl). 2013;125:523–33.
Georges A, Gros P, Fodil N. USP15: a review of its implication in immune and inflammatory processes and tumor progression. Genes Immun. 2021;22:12–23.
Yeh H-M, Yu C-Y, Yang H-C, Ko S-H, Liao C-L, Lin Y-L. Ubiquitin-specific protease 13 regulates IFN signaling by stabilizing STAT1. J Immunol. 2013;191:3328–36.
Riccio V, Demers N, Hua R, Vissa M, Cheng DT, Strilchuk AW, et al. Deubiquitinating enzyme USP30 maintains basal peroxisome abundance by regulating pexophagy. J Cell Biol. 2019;218:798–807.
Chaudhary S, Behari M, Dihana M, Swaminath PV, Govindappa ST, Jayaram S, et al. Parkin mutations in familial and sporadic Parkinson’s disease among Indians. Parkinsonism Relat Disord. 2006;12:239–45.
Klein C, Lohmann K. Parkinson disease(s): is “parkin disease” a distinct clinical entity? Neurology. 2009;72:106–7.
Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;69:2093–104.
Pilcher H. Parkin implicated in sporadic Parkinson’s disease. Lancet Neurol. 2005;4:798.
Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14:463–77.
Tran J, Anastacio H, Bardy C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. NPJ Parkinsons Dis. 2020;6:8.
Brahmachari S, Ge P, Lee SH, Kim D, Karuppagounder SS, Kumar M, et al. Activation of tyrosine kinase c-Abl contributes to α-synuclein-induced neurodegeneration. J Clin Invest. 2016;126:2970–88.
Acknowledgments
The authors thank Noelle Burgess for assistance in creating the figures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NS38377, NS097049, and NS102035), and the JPB Foundation.
Conflicts of interest/competing interests
Consulting: T.M.D has received personal compensation for consulting with Sun Pharma Advanced Research Company; T.M.D. is a consultant for Mitokinin; T.M.D. and V.L.D. are consultants to Inhibikase Therapeutics Inc.; T.M.D. serves on the Board of Directors and is compensated for his roles as a consultant and interim Chief Scientific Officer of Valted Seq Inc.; V.L.D. serves on the Board of Directors and is compensated for her role as interim Chief Executive Officer of Valted Seq Inc. T.M.D. was a paid consultant and advisory board member to DONG-A ST; T.M.D. and V.L.D. received personal compensation for consulting and serving on a scientific advisory board with Hopstem, Inc. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Stock Ownership: T.M.D. owns stock options in American Gene Technologies International Inc.; T.M.D owns stock and stock options in Mitokinin; T.M.D. and V.L.D. own stock options in Inhibikase Therapeutics Inc.; T.M.D. and V.L.D. are founders of Valted, LLC and holds an ownership equity interest in the company. T.M.D. and V.L.D. are inventors of technology of Neuraly, Inc. that has optioned from Johns Hopkins University. T.M.D. and V.L.D. are founders of, and holds shares of stock options as well as equity in, Neuraly, Inc., which is now a subsidiary of D & D Pharmatech; T.M.D. and V.L.D. holds shares of stock options as well as equity in D & D Pharmatech; T.M.D. and V.L.D. are founders of and hold equity in Valted Seq Inc.; these arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. J.P. and N.P. have no conflicts of interest or disclosures.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
No original data or supplemental materials were generated in the process of writing this manuscript.
Code availability
Not applicable.
Author contributions
JP reviewed the literature, conceptualized and developed the original draft of the manuscript, and participated in reviewing as well as editing the manuscript. NP participated in the literature searching and conceptualizing, reviewing, and editing the manuscript. TMD and VLD both participated in reviewing, editing, and conceptualizing the manuscript. All authors have read and approved the final version of the manuscript, and they agree to be accountable for the work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, J., Panicker, N., Dawson, V.L. et al. Cell Biology of Parkin: Clues to the Development of New Therapeutics for Parkinson’s Disease. CNS Drugs 36, 1249–1267 (2022). https://doi.org/10.1007/s40263-022-00973-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00973-7