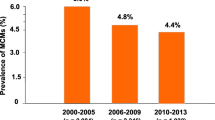
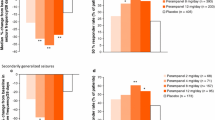
Abstract
Drug-resistant epilepsy is associated with poor health outcomes and increased economic burden. In the last three decades, various new antiseizure medications have been developed, but the proportion of people with drug-resistant epilepsy remains relatively unchanged. Develo** strategies to address drug-resistant epilepsy is essential. Here, we define drug-resistant epilepsy and emphasize its relationship to the conceptualization of epilepsy as a symptom complex, delineate clinical risk factors, and characterize mechanisms based on current knowledge. We address the importance of ruling out pseudoresistance and consider the impact of nonadherence on determining whether an individual has drug-resistant epilepsy. We then review the principles of epilepsy drug therapy and briefly touch upon newly approved and experimental antiseizure medications.
Similar content being viewed by others
References
Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303.
Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia. 2018;59(12):2179–93.
Xue-** W, Hai-Jiao W, Li-Na Z, Xu D, Ling L. Risk factors for drug-resistant epilepsy: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(30): e16402.
Hernández-Ronquillo L, Adams S, Ballendine S, Téllez-Zenteno JF. Epilepsy in an elderly population: classification, etiology and drug resistance. Epilepsy Res. 2018;140:90–4.
Sultana B, Panzini M-A, Carpentier AV, et al. Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology. 2021;96(17):805–17.
Mohanraj R, Norrie J, Stephen LJ, Kelly K, Hitiris N, Brodie MJ. Mortality in adults with newly diagnosed and chronic epilepsy: a retrospective comparative study. Lancet Neurol. 2006;5(6):481–7.
Lawn ND, Bamlet W, Radhakrishnan K, O’Brien P, So EL. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565–70.
McCagh J, Fisk JE, Baker GA. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009;86(1):1–14.
Akdemir V, Sut N, Guldiken B. Factors affecting the quality of life in drug-resistant epilepsy patients. Acta Neurol Belg. 2016;116(4):513–8.
Ridsdale L, Wojewodka G, Robinson E, et al. Characteristics associated with quality of life among people with drug-resistant epilepsy. J Neurol. 2017;264(6):1174–84.
Cramer JA, Wang ZJ, Chang E, et al. Healthcare utilization and costs in adults with stable and uncontrolled epilepsy. Epilepsy Behav. 2014;31:356–62.
Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies [published correction appears in Epilepsia. 2010;51(9):1922]. Epilepsia. 2010;51(6):1069–77. https://doi.org/10.1111/j.1528-1167.2009.02397.x
Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919–26.
Nair DR. Management of drug-resistant epilepsy. Continuum (Minneap Minn). 2016;22(1):157–72.
Arts WF, Brouwer OF, Peters AB, et al. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127(8):1774–84.
Geerts A, Arts WF, Stroink H, et al. Course and outcome of childhood epilepsy: a 15-year follow-up of the Dutch Study of Epilepsy in Childhood. Epilepsia. 2010;51(7):1189–97.
Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–86.
Hao X-T, Wong IS, Kwan P. Interrater reliability of the international consensus definition of drug-resistant epilepsy: a pilot study. Epilepsy Behav. 2011;22(2):388–90.
Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. 2010;9(1):27–9.
Shlobin NA, Sander JW. Learning from the comorbidities of epilepsy. Curr Opin Neurol. 2022;35(2):175–80.
Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–15.
Neligan A, Hauser WA, Sander JW. The epidemiology of the epilepsies. Handb Clin Neurol. 2012;107:113–33.
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701.
Fisher RS, Boas WVE, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–2.
England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav. 2012;25(2):266–76.
Sillanpää M, Schmidt D. Early seizure frequency and aetiology predict long-term medical outcome in childhood-onset epilepsy. Brain. 2009;132(4):989–98.
Ramos-Lizana J, Aguilera-López P, Aguirre-Rodríguez J, Cassinello-García E. Response to sequential treatment schedules in childhood epilepsy: risk for development of refractory epilepsy. Seizure. 2009;18(9):620–4.
Karaoğlu P, Yiş U, Polat Aİ, Ayanoğlu M, Hiz AS. Clinical predictors of drug-resistant epilepsy in children. Turk J Med Sci. 2021;51(3):1249–52.
Boonluksiri P, Visuthibhan A, Katanyuwong K. Clinical prediction rule of drug resistant epilepsy in children. J Epilepsy Res. 2015;5(2):84.
Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia. 2008;49(7):1230–8.
Gilioli I, Vignoli A, Visani E, et al. Focal epilepsies in adult patients attending two epilepsy centers: classification of drug-resistance, assessment of risk factors, and usefulness of “new” antiepileptic drugs. Epilepsia. 2012;53(4):733–40.
Roy PL, Ronquillo LH, Ladino LD, Tellez-Zenteno JF. Risk factors associated with drug resistant focal epilepsy in adults: a case control study. Seizure. 2019;73:46–50.
Jeong A, Nakagawa JA, Wong M. Predictors of drug-resistant epilepsy in tuberous sclerosis complex. J Child Neurol. 2017;32(14):1092–8.
Gelisse P, Genton P, Thomas P, Rey M, Samuelian J, Dravet C. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):240–3.
Delen D, Davazdahemami B, Eryarsoy E, Tomak L, Valluru A. Using predictive analytics to identify drug-resistant epilepsy patients. Health Inform J. 2020;26(1):449–60.
An S, Malhotra K, Dilley C, et al. Predicting drug-resistant epilepsy: a machine learning approach based on administrative claims data. Epilepsy Behav. 2018;89:118–25.
Semah F, Picot M-C, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256–62.
Löscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020;72(3):606–38.
Sisodiya SM. Mechanisms of antiepileptic drug resistance. Curr Opin Neurol. 2003;16(2):197–201.
Margineanu DG, Klitgaard H. Mechanisms of drug resistance in epilepsy: relevance for antiepileptic drug discovery. Expert Opin Drug Discov. 2009;4(1):23–32.
Dalic L, Cook MJ. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat. 2016;12:2605.
Tang F, Hartz A, Bauer B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol. 2017;8:301.
Shorvon WL, Schmidt D. Mechanisms of drug resistance and tolerance. In: Shorvon S, Perucca E, Engel J (eds) The treatment of epilepsy. 2015. https://doi.org/10.1002/9781118936979.ch7
Rogawski MA, Löscher W, Rho JM. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harbor Perspect Med. 2016;6(5): a022780.
Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129(1):18–35.
Remy S, Gabriel S, Urban BW, et al. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53(4):469–79.
Remy S, Urban BW, Elger CE, Beck H. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. Eur J Neurosci. 2003;17(12):2648–58.
Schaub C, Uebachs M, Beck H. Diminished response of CA1 neurons to antiepileptic drugs in chronic epilepsy. Epilepsia. 2007;48(7):1339–50.
Catterall WA. Sodium channel mutations and epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. 4th edn. Bethesda, MD: National Center for Biotechnology Information (US); 2012.
Kaplan DI, Isom LL, Petrou S. Role of sodium channels in epilepsy. Cold Spring Harbor Perspect Med. 2016;6(6): a022814.
Rusconi R, Combi R, Cestèle S, et al. A rescuable folding defective Nav1.1 (SCN1A) sodium channel mutant causes GEFS+: common mechanism in Nav1.1 related epilepsies? Hum Mutat. 2009;30(7):E747–60.
Rusconi R, Scalmani P, Cassulini RR, et al. Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J Neurosci. 2007;27(41):11037–46.
Lucas PT, Meadows LS, Nicholls J, Ragsdale DS. An epilepsy mutation in the β1 subunit of the voltage-gated sodium channel results in reduced channel sensitivity to phenytoin. Epilepsy Res. 2005;64(3):77–84.
Sheilabi MA, Takeshita LY, Sims EJ, Falciani F, Princivalle AP. The sodium channel B4-subunits are dysregulated in temporal lobe epilepsy drug-resistant patients. Int J Mol Sci. 2020;21(8):2955.
Zamponi GW, Lory P, Perez-Reyes E. Role of voltage-gated calcium channels in epilepsy. Pflügers Arch. 2010;460(2):395–403.
Cain SM, Snutch TP. Voltage-gated calcium channels in epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies. 4th edn. Bethesda, MD: National Center for Biotechnology Information (US); 2012.
Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67(4):821–70.
Vitko I, Bidaud I, Arias JM, Mezghrani A, Lory P, Perez-Reyes E. The I-II loop controls plasma membrane expression and gating of Cav3.2 T-type Ca2+ channels: a paradigm for childhood absence epilepsy mutations. J Neurosci. 2007;27(2):322–30.
Heron SE, Khosravani H, Varela D, et al. Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann Neurol. 2007;62(6):560–8.
Khosravani H, Bladen C, Parker DB, Snutch TP, McRory JE, Zamponi GW. Effects of Cav3.2 channel mutations linked to idiopathic generalized epilepsy. Ann Neurol. 2005;57(5):745–9.
Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011;2011: 297153.
Shulga A, Thomas-Crusells J, Sigl T, et al. Posttraumatic GABAA-mediated [Ca2+] i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J Neurosci. 2008;28(27):6996–7005.
Juvale IIA, Che Has AT. Possible interplay between the theories of pharmacoresistant epilepsy. Eur J Neurosci. 2021;53(6):1998–2026.
Frei MG, Zaveri HP, Arthurs S, et al. Controversies in epilepsy: debates held during the Fourth International Workshop on Seizure Prediction. Epilepsy Behav. 2010;19(1):4–16.
Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18(5):467–86.
Goetz T, Arslan A, Wisden W, Wulff P. GABAA receptors: structure and function in the basal ganglia. Progress Brain Res. 2007;160:21–41.
Blair RE, Sombati S, Churn SB, DeLorenzo RJ. Epileptogenesis causes an N-methyl-d-aspartate receptor/Ca2+-dependent decrease in Ca2+/calmodulin-dependent protein kinase II activity in a hippocampal neuronal culture model of spontaneous recurrent epileptiform discharges. Eur J Pharmacol. 2008;588(1):64–71.
Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008;28(10):2527–38.
Joshi S, Rajasekaran K, Hawk KM, et al. Phosphatase inhibition prevents the activity-dependent trafficking of GABAA receptors during status epilepticus in the young animal. Epilepsia. 2015;56(9):1355–65.
Majoie HM, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res. 2006;71(2–3):135–41.
Fonseca-Barriendos D, Frías-Soria CL, Pérez-Pérez D, Gómez-López R, Borroto Escuela DO, Rocha L. Drug-resistant epilepsy: drug target hypothesis and beyond the receptors. Epilepsia Open. 2021. https://doi.org/10.1002/epi4.12539.
Janmohamed M, Brodie MJ, Kwan P. Pharmacoresistance: epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology. 2020;168: 107790.
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36(1):1–6.
Saunders NR, Habgood MD, Møllgård K, Dziegielewska KM. The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system? F1000Research. 2016;5:313.
Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metabol Dis. 2013;36(3):437–49.
Sisodiya S, Martinian L, Scheffer G, et al. Vascular colocalization of P-glycoprotein, multidrug-resistance associated protein 1, breast cancer resistance protein and major vault protein in human epileptogenic pathologies. Neuropathol Appl Neurobiol. 2006;32(1):51–63.
Sisodiya S, Lin WR, Harding B, Squier M, Thom M. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain. 2002;125(1):22–31.
Feldmann M, Koepp M. P-glycoprotein imaging in temporal lobe epilepsy: in vivo PET experiments with the Pgp substrate [11C]-verapamil. Epilepsia. 2012;53:60–3.
Shin J-W, Chu K, Shin S, et al. Clinical applications of simultaneous PET/MR imaging using (R)-[11C]-verapamil with cyclosporin A: preliminary results on a surrogate marker of drug-resistant epilepsy. Am J Neuroradiol. 2016;37(4):600–6.
Ilyas-Feldmann M, Asselin MC, Wang S, et al. P-glycoprotein overactivity in epileptogenic developmental lesions measured in vivo using (R)-[11C] verapamil PET. Epilepsia. 2020;61(7):1472–80.
Borlot F, Wither RG, Ali A, Wu N, Verocai F, Andrade DM. A pilot double-blind trial using verapamil as adjuvant therapy for refractory seizures. Epilepsy Res. 2014;108(9):1642–51.
Van Vliet E, Aronica E, Gorter J. Blood–brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol. 2015;38:26–34.
Zhang C, Kwan P. The concept of drug-resistant epileptogenic zone. Front Neurol. 2019;10:558.
Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, D’Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48:140–9.
Ghosh C, Puvenna V, Gonzalez-Martinez J, Janigro D, Marchi N. Blood–brain barrier P450 enzymes and multidrug transporters in drug resistance: a synergistic role in neurological diseases. Curr Drug Metab. 2011;12(8):742–9.
Pitkänen A, Buckmaster P, Galanopoulou AS, Moshé SL. Models of seizures and epilepsy. New York: Academic Press; 2017.
Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr. 2008;8(5):127–30.
Rogawski MA. The intrinsic severity hypothesis of pharmacoresistance to antiepileptic drugs. Epilepsia. 2013;54:33–40.
Löscher W, Brandt C. High seizure frequency prior to antiepileptic treatment is a predictor of pharmacoresistant epilepsy in a rat model of temporal lobe epilepsy. Epilepsia. 2010;51(1):89–97.
Schmidt D, Löscher W. New developments in antiepileptic drug resistance: an integrative view. Epilepsy Curr. 2009;9(2):47–52.
Fang M, ** Z-Q, Wu Y, Wang X-F. A new hypothesis of drug refractory epilepsy: neural network hypothesis. Med Hypotheses. 2011;76(6):871–6.
Barkovich AJ, Dobyns WB, Guerrini R. Malformations of cortical development and epilepsy. Cold Spring Harbor Perspect Med. 2015;5(5): a022392.
Paolini S, Morace R, Di Gennaro G, et al. Drug-resistant temporal lobe epilepsy due to cavernous malformations. Neurosurg Focus. 2006;21(1):1–5.
Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8(12):669–77.
Volk HA, Arabadzisz D, Fritschy J-M, Brandt C, Bethmann K, Löscher W. Antiepileptic drug-resistant rats differ from drug-responsive rats in hippocampal neurodegeneration and GABAA receptor ligand binding in a model of temporal lobe epilepsy. Neurobiol Dis. 2006;21(3):633–46.
Bethmann K, Fritschy J-M, Brandt C, Löscher W. Antiepileptic drug resistant rats differ from drug responsive rats in GABAA receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis. 2008;31(2):169–87.
Roitbak T, Syková E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28(1):40–8.
Robel S, Buckingham SC, Boni JL, et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci. 2015;35(8):3330–45.
Galovic M, Baudracco I, Wright-Goff E, et al. Association of piriform cortex resection with surgical outcomes in patients with temporal lobe epilepsy. JAMA Neurol. 2019;76(6):690–700.
Balestrini S, Sisodiya SM. Pharmacogenomics in epilepsy. Neurosci Lett. 2018;667:27–39.
Lopez-Garcia MA, Feria-Romero IA, Fernando-Serrano H, Escalante-Santiago D, Grijalva I, Orozco-Suarez S. Genetic polymorphisms associated with antiepileptic metabolism. Front Biosci (Elite Ed). 2014;6:377–86.
Depondt C, Godard P, Espel RS, Da Cruz A, Lienard P, Pandolfo M. A candidate gene study of antiepileptic drug tolerability and efficacy identifies an association of CYP2C9 variants with phenytoin toxicity. Eur J Neurol. 2011;18(9):1159–64.
Mirza N, Stevelink R, Taweel B, Koeleman BP, Marson AG. Using common genetic variants to find drugs for common epilepsies. Brain Commun. 2021;3(4):fcab287.
Wolking S, Moreau C, McCormack M, et al. Assessing the role of rare genetic variants in drug-resistant, non-lesional focal epilepsy. Ann Clin Transl Neurol. 2021;8(7):1376–87.
Gambardella A, Labate A, Mumoli L, Lopes-Cendes I, Cendes F. Role of pharmacogenomics in antiepileptic drug therapy: current status and future perspectives. Curr Pharm Design. 2017;23(37):5760–5.
Kobow K, Blümcke I. Epigenetics in epilepsy. Neurosci Lett. 2018;667:40–6.
Kobow K, El-Osta A, Blümcke I. The methylation hypothesis of pharmacoresistance in epilepsy. Epilepsia. 2013;54:41–7.
Haenisch S, von Rüden E-L, Wahmkow H, et al. miRNA-187-3p-mediated regulation of the KCNK10/TREK-2 potassium channel in a rat epilepsy model. ACS Chem Neurosci. 2016;7(11):1585–94.
Morris G, Reschke CR, Henshall DC. Targeting microRNA-134 for seizure control and disease modification in epilepsy. EBioMedicine. 2019;45:646–54.
Bohosova J, Vajcner J, Jabandziev P, Oslejskova H, Slaby O, Aulicka S. MicroRNAs in the development of resistance to antiseizure drugs and their potential as biomarkers in pharmacoresistant epilepsy. Epilepsia. 2021;62(11):2573–88.
De Benedittis S, Fortunato F, Cava C, et al. Circulating microRNAs as potential novel diagnostic biomarkers to predict drug resistance in temporal lobe epilepsy: a pilot study. Int J Mol Sci. 2021;22(2):702.
Holmes M, Flaminio Z, Vardhan M, et al. Cross talk between drug-resistant epilepsy and the gut microbiome. Epilepsia. 2020;61(12):2619–28.
Chatzikonstantinou S, Gioula G, Kimiskidis VK, McKenna J, Mavroudis I, Kazis D. The gut microbiome in drug-resistant epilepsy. Epilepsia Open. 2021;6(1):28–37.
Peng A, Qiu X, Lai W, et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 2018;147:102–7.
Lee H, Lee S, Lee D-H, Kim DW. A comparison of the gut microbiota among adult patients with drug-responsive and drug-resistant epilepsy: an exploratory study. Epilepsy Res. 2021;172: 106601.
Cheraghmakani H, Rezai MS, Valadan R, et al. Ciprofloxacin for treatment of drug-resistant epilepsy. Epilepsy Res. 2021;176: 106742.
Gómez-Eguílaz M, Ramón-Trapero J, Pérez-Martínez L, Blanco J. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef Microbes. 2018;9(6):875–81.
Friedman A, Heinemann U. Role of blood-brain barrier dysfunction in epileptogenesis. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th edn. Bethesda, MD: National Center for Biotechnology Information (US); 2012.
Bazhanova ED, Kozlov AA, Litovchenko AV. Mechanisms of drug resistance in the pathogenesis of epilepsy: role of neuroinflammation. A literature review. Brain Sci. 2021;11(5):663.
Salar S, Maslarova A, Lippmann K, et al. Blood–brain barrier dysfunction can contribute to pharmacoresistance of seizures. Epilepsia. 2014;55(8):1255–63.
Lerche H. Drug-resistant epilepsy: time to target mechanisms. Nat Rev Neurol. 2020;16(11):595–6.
Baraban SC, Löscher W. What new modeling approaches will help us identify promising drug treatments? Adv Exp Med Biol. 2014;813:283–94.
Samsonsen C, Reimers A, Bråthen G, Helde G, Brodtkorb E. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia. 2014;55(11):e125–8.
Malek N, Heath C, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507–15.
O’Rourke G, O’Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160–8.
Ferrari CMM, de Sousa RMC, Castro LH. Factors associated with treatment non-adherence in patients with epilepsy in Brazil. Seizure. 2013;22(5):384–9.
Getachew H, Dekema N, Awol S, Abdi A, Mohammed M. Medication adherence in epilepsy and potential risk factors associated with non adherence in tertiary care teaching hospital in southwest Ethiopia. Gaziantep Med J. 2014;20(1):59–65.
Henning O, Johannessen Landmark C, Nakken KO, Lossius MI. Nonadherence to treatment regimens in epilepsy from the patient’s perspective and predisposing factors: differences between intentional and unintentional lack of adherence. Epilepsia. 2019;60(5):e58-62.
Henning O, Lossius MI, Lima M, et al. Refractory epilepsy and nonadherence to drug treatment. Epilepsia Open. 2019;4(4):618–23.
Getnet A, Woldeyohannes SM, Bekana L, et al. Antiepileptic drug nonadherence and its predictors among people with epilepsy. Behav Neurol. 2016;2016:3189108.
Paschal AM, Rush SE, Sadler T. Factors associated with medication adherence in patients with epilepsy and recommendations for improvement. Epilepsy Behav. 2014;31:346–50.
Tang F, Zhu G, Jiao Z, Ma C, Wang B. Self-reported adherence in patients with epilepsy who missed their medications and reasons for nonadherence in China. Epilepsy Behav. 2013;27(1):85–9.
Chapman SC, Horne R, Chater A, Hukins D, Smithson WH. Patients’ perspectives on antiepileptic medication: relationships between beliefs about medicines and adherence among patients with epilepsy in UK primary care. Epilepsy Behav. 2014;31:312–20.
Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372–8.
Paschal AM, Hawley SR, Romain TS, Ablah E. Measures of adherence to epilepsy treatment: review of present practices and recommendations for future directions. Epilepsia. 2008;49(7):1115–22.
Graves N, Holmes G, Leppik I. Compliant populations: variability in serum concentrations. Epilepsy Res Suppl. 1988;1:91–9.
Tomson T, Dahl ML, Kimland E. Therapeutic monitoring of antiepileptic drugs for epilepsy. Cochrane Database Syst Rev. 2007;2007(1):CD002216.
Malone SA, Eadie MJ, Addison RS, Wright AW, Dickinson RG. Monitoring salivary lamotrigine concentrations. J Clin Neurosci. 2006;13(9):902–7.
Grim SA, Ryan M, Miles MV, et al. Correlation of levetiracetam concentrations between serum and saliva. Ther Drug Monit. 2003;25(1):61–6.
Al Za M, Deleu D, Batchelor C. Salivary free concentrations of anti-epileptic drugs: an evaluation in a routine clinical setting. Acta Neurol Belg. 2003;103:19–23.
Dwivedi R, Singh M, Kaleekal T, Gupta YK, Tripathi M. Concentration of antiepileptic drugs in persons with epilepsy: a comparative study in serum and saliva. Int J Neurosci. 2016;126(11):972–8.
Kintz P, Marescaux C, Mangin P. Testing human hair for carbamazepine in epileptic patients: is hair investigation suitable for drug monitoring? Hum Exp Toxicol. 1995;14(10):812–5.
Williams J, Myson V, Steward S, et al. Self-discontinuation of antiepileptic medication in pregnancy: detection by hair analysis. Epilepsia. 2002;43(8):824–31.
Williams J, Patsalos PN, Mei Z, Schapel G, Wilson JF, Richens A. Relation between dosage of carbamazepine and concentration in hair and plasma samples from a compliant inpatient epileptic population. Ther Drug Monitor. 2001;23(1):15–20.
Kuczynska J, Karas-Ruszczyk K, Zakrzewska A, et al. Comparison of plasma, saliva, and hair lamotrigine concentrations. Clin Biochem. 2019;74:24–30.
Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the quality of life in epilepsy inventory for adolescents: the QOLIE-AD-48. Epilepsia. 1999;40(8):1114–21.
Pakpour AH, Gholami M, Esmaeili R, et al. A randomized controlled multimodal behavioral intervention trial for improving antiepileptic drug adherence. Epilepsy Behav. 2015;52:133–42.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74.
McAuley JW, McFadden LS, Elliott JO, Shneker BF. An evaluation of self-management behaviors and medication adherence in patients with epilepsy. Epilepsy Behav. 2008;13(4):637–41.
DiIorio C, Hennessy M, Manteuffel B. Epilepsy self-management: a test of a theoretical model. Nurs Res. 1996;45(4):211–7.
Dilorio C, Faherty B, Manteuffel B, Hoeffer B, Hilbert GA. Self-efficacy and social support in self-management of epilepsy. West J Nurs Res. 1992;14(3):292–307.
Dilorio C, Faherty B. Epilepsy self-management: partial replication and extension. Res Nurs Health. 1994;17(3):167–74.
DiIorio C, Shafer PO, Letz R, et al. Behavioral, social, and affective factors associated with self-efficacy for self-management among people with epilepsy. Epilepsy Behav. 2006;9(1):158–63.
Lisk D, Greene S. Drug compliance and seizure control in epileptic children. Postgrad Med J. 1985;61(715):401–5.
Mitchell WG, Scheier LM, Baker SA. Adherence to treatment in children with epilepsy: who follows" doctor’s orders"? Epilepsia. 2000;41(12):1616–25.
Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261(22):3273–7.
Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26:814–23.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Singh P, Gupta K, Singh G, Kaushal S. Simultaneous use of two different tools to assess compliance with antiepileptic drugs: experience in a community-based study. J Neurosci Rural Pract. 2020;11(04):636–9.
Al-aqeel S, Al-sabhan J. Strategies for improving adherence to antiepileptic drug treatment in patients with epilepsy. Cochrane Database Syst Rev. 2011;1:CD008312.
Dash D, Sebastian TM, Aggarwal M, Tripathi M. Impact of health education on drug adherence and self-care in people with epilepsy with low education. Epilepsy Behav. 2015;44:213–7.
AlAjmi R, Al-Aqeel S, Baz S. The impact of a pharmacist-led educational interview on medication adherence of Saudi patients with epilepsy. Patient Prefer Adher. 2017;11:959.
Tang F, Zhu G, Jiao Z, Ma C, Chen N, Wang B. The effects of medication education and behavioral intervention on Chinese patients with epilepsy. Epilepsy Behav. 2014;37:157–64.
Sancho J, Peña P, Rufo M, Palacios G, Masramon X, Rejas J. Health and non-health care resources use in the management of adult outpatients with drug-resistant epilepsy in Spain: a cost-of-illness study (LINCE study). Epilepsy Res. 2008;81(2–3):176–87.
Owczarek K, Jedrzejczak J. Economic aspects of drug-resistant epilepsy. Neurol Neurochir Pol. 2001;35(2):309–18.
Sunny AA, Iyer RS, Kumaran SG, Bunshaw NG, Shanmugham K, Govindaraj U. Affordability, availability and tolerability of antiseizure medications are better predictors of adherence than beliefs: changing paradigms from a low resource setting. Seizure. 2020;83:208–15.
von Gaudecker JR, Buelow JM, Miller WR, Tanner AL, Austin JK. Social determinants of health associated with epilepsy treatment adherence in the United States: a sco** review. Epilepsy Behav. 2021;124: 108328.
Teh KX, Henien NPB, Wong LS, et al. A cross-sectional study on the rate of non-adherence to antiseizure medications and factors associated with non-adherence among patients with epilepsy. PLoS ONE. 2020;15(7): e0235674.
Groenewegen A, Tofighy A, Ryvlin P, Steinhoff BJ, Dedeken P. Measures for improving treatment outcomes for patients with epilepsy: results from a large multinational patient-physician survey. Epilepsy Behav. 2014;34:58–67.
Sander J. Ultimate success in epilepsy: the patient’s perspective. Eur J Neurol. 2005;12(Suppl. 4):3–11.
Rugg-Gunn FJ, Sander JW. Management of chronic epilepsy. BMJ. 2012;345: e4576.
Shlobin NA, Clark JR, Hoffman SC, Hopkins BS, Kesavabhotla K, Dahdaleh NS. Patient education in neurosurgery: Part 1 of a systematic review. World Neurosurg. 2021;147(190–201): e1.
Shlobin NA, Clark JR, Hoffman SC, Hopkins BS, Kesavabhotla K, Dahdaleh NS. Patient education in neurosurgery: Part 2 of a systematic review. World Neurosurg. 2021;147:190-201.e1.
Sander JW. The use of antiepileptic drugs: principles and practice. Epilepsia. 2004;45:28–34.
Brodie MJ, Kwan P. The star systems. CNS Drugs. 2001;15(1):1–12.
Haneef Z, Stern J, Dewar S, Engel J. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75(8):699–704.
Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47(6):952–9.
Shlobin NA, Campbell JM, Rosenow JM, Rolston JD. Ethical considerations in the surgical and neuromodulatory treatment of epilepsy. Epilepsy Behav. 2022;127: 108524.
Shlobin NA, Rosenow JM. Ethical considerations in the implantation of neuromodulatory devices. Neuromodulation. 2021. https://doi.org/10.1111/ner.13357.
Shlobin NA, Sheldon M, Lam S. Informed consent in neurosurgery: a systematic review. Neurosurg Focus. 2020;49(5):E6.
Epilepsy Foundation. FDA news: cenobamate (XCOPRI®) for focal epilepsy in adults. Available from: https://www.epilepsy.com/article/2019/12/fda-news-cenobamate-xcopri%C2%AE-focal-epilepsy-adults. Accessed 7 Jan 2022.
Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48.
Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–22.
Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. 2020;61(6):1099–108.
Lattanzi S, Trinka E, Zaccara G, et al. Adjunctive cenobamate for focal-onset seizures in adults: a systematic review and meta-analysis. CNS Drugs. 2020;34(11):1105–20.
Sander JW, Rosenfeld WE, Halford JJ, Steinhoff BJ, Biton V, Toledo M. Long-term individual retention with cenobamate in adults with focal seizures: pooled data from the clinical development program. Epilepsia. 2022;63(1):139–49.
Sharma R, Nakamura M, Neupane C, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879: 173117.
Guignet M, Campbell A, White HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. 2020;61(11):2329–39.
Gogou M, Cross JH. Fenfluramine as antiseizure medication for epilepsy. Dev Med Child Neurol. 2021;63(8):899–907.
Lagae L, Sullivan J, Knupp K, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10216):2243–54.
Nabbout R, Mistry A, Zuberi S, et al. Fenfluramine for treatment-resistant seizures in patients with Dravet syndrome receiving stiripentol-inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77(3):300–8.
Lagae L, Schoonjans AS, Gammaitoni AR, Galer BS, Ceulemans B. A pilot, open-label study of the effectiveness and tolerability of low-dose ZX 008 (fenfluramine HC l) in Lennox–Gastaut syndrome. Epilepsia. 2018;59(10):1881–8.
Billakota S, Devinsky O, Marsh E. Cannabinoid therapy in epilepsy. Curr Opin Neurol. 2019;32(2):220–6.
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–8.
Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–20.
Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–11.
Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox–Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–96.
Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378(20):1888–97.
Privitera M, Bhathal H, Wong M, et al. Time to onset of cannabidiol (CBD) treatment effect in Lennox–Gastaut syndrome: analysis from two randomized controlled trials. Epilepsia. 2021;62(5):1130–40.
Madan Cohen J, Checketts D, Dunayevich E, et al. Time to onset of cannabidiol treatment effects in Dravet syndrome: analysis from two randomized controlled trials. Epilepsia. 2021;62(9):2218–27.
Bialer M, Perucca E. Does cannabidiol have antiseizure activity independent of its interactions with clobazam? An appraisal of the evidence from randomized controlled trials. Epilepsia. 2020;61(6):1082–9.
Wiegand G, May TW, Ostertag P, Boor R, Stephani U, Franz DN. Everolimus in tuberous sclerosis patients with intractable epilepsy: a treatment option? Eur J Paediatr Neurol. 2013;17(6):631–8.
Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74(5):679–87.
Krueger DA, Wilfong AA, Mays M, et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology. 2016;87(23):2408–15.
French JA, Lawson JA, Yapici Z, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153–63.
Overwater IE, Rietman AB, van Eeghen AM, de Wit MCY. Everolimus for the treatment of refractory seizures associated with tuberous sclerosis complex (TSC): current perspectives. Ther Clin Risk Manag. 2019;15:951.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Nathan A. Shlobin received no funding. Josemir W. Sander is based at the NIHR University College London Hospitals Biomedical Research Centre, which receives a proportion of funding from the UK Department of Health’s Research Centres funding scheme. He receives research support from the Dr Marvin Weil Epilepsy Research Fund, from the UK Epilepsy Society, and the Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie, the Netherlands.
Conflict of interest
Nathan A. Shlobin has no disclosures to report. Josemir W. Sander reports personal fees from Eisai, UCB, GW, Arvelle, and Zogenix, and grants from UCB outside the submitted work.
Ethics approval
Not required or sought. This was a narrative review of existing literature.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All relevant data can be found in the references of this review.
Code availability
Not applicable.
Author contributions
NAS conducted the literature search and data analysis and drafted the manuscript. JWS formulated the concept for the article and provided critical intellectual input. Both authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Rights and permissions
About this article
Cite this article
Shlobin, N.A., Sander, J.W. Current Principles in the Management of Drug-Resistant Epilepsy. CNS Drugs 36, 555–568 (2022). https://doi.org/10.1007/s40263-022-00922-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00922-4