Abstract
Background and Objective
Pediatric dosing of enoxaparin was derived based on extrapolation of the adult therapeutic range to children. However, a large fraction of children do not achieve therapeutic anticoagulation with initial dosing. We aim to use real-world anti-Xa data obtained from children receiving enoxaparin per standard of care to characterize the population pharmacokinetics (PopPK).Author names: Please confirm if the author names are presented accurately and in the correct sequence (given name, middle name/initial, family name). Also, kindly confirm the details in the metadata are correct.The author names are accurately presented and the metadata are correct.
Methods
A PopPK analysis was performed using NONMEM, and a stepwise covariate modeling approach was applied for the covariate selection. The final PopPK model, developed with data from 1293 patients ranging in age from 1 day to 18 years, was used to simulate enoxaparin subcutaneous dosing for prophylaxis and treatment based on total body weight (0–18 years, TBW) or fat-free mass (2–18 years, FFM). Simulated exposures in children with obesity (body mass index percentile ≥95th percentile) were compared with those without obesity.
Results
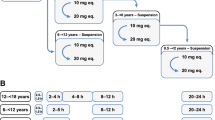
A linear, one-compartment PopPK model that included allometric scaling using TBW (<2 years) or FFM (≥2 years) characterized the enoxaparin pharmacokinetic data. In addition, serum creatinine was identified as a significant covariate influencing clearance. Simulations indicated that in patients aged <2 years, the recommended 1.5 mg/kg TBW-based dosing achieves therapeutic simulated concentrations. In pediatric patients aged ≥2 years, the recommended 1.0 mg/kg dose resulted in exposures more comparable in children with and without obesity when FFM weight-based dosing was applied.
Conclusion
Using real-world data and PopPK modeling, enoxaparin’s pharmacokinetics were characterized in pediatric patients. Using FFM and twice-daily dosing might reduce the risk of overdosing, especially in children with obesity.





Similar content being viewed by others
References
Lovenox [product monograph] [Internet]. 2018. Available from https://pdf.hres.ca/dpd_pm/00047708.PDF
Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, et al. Antithrombotic therapy in neonates and children. Chest. 2012;141:e737S-e801S.
Schobess R, Düring C, Bidlingmaier C, Heinecke A, Merkel N, Nowak-Göttl U. Long-term safety and efficacy data on childhood venous thrombosis treated with a low molecular weight heparin: an open-label pilot study of once-daily versus twice-daily enoxaparin administration. Haematologica. 2006;91:1701–4.
Dix D, Andrew M, Marzinotto V, Charpentier K, Bridge S, Monagle P, et al. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136:439–45.
Anticoagulation T, Leung M, Ho SH, Hamilton DP. JPPT Clinical investigation utility of anti-Xa monitoring in children receiving enoxaparin for therapeutic anticoagulation. Pediatr Pharmacol. 2005;10(1):43–50.
Fung LS, Klockau C. Effects of age and weight-based dosing of enoxaparin on anti-factor Xa levels in pediatric patients. J Pediatr Pharmacol Ther. 2010;15:119–25.
Trame MN, Mitchell L, Krümpel A, Male C, Hempel G, Nowak-Göttl U. Population pharmacokinetics of enoxaparin in infants, children and adolescents during secondary thromboembolic prophylaxis: a cohort study. J Thromb Haemost. 2010;8:1950–8.
Monagle P, Michelson AD, Bovill E, Andrew M. Antithrombotic therapy in children. Chest. 2001;119:344S-370S.
Albisetti M, Andrew M. Low molecular weight heparin in children. Eur J Pediatr. 2002;161:71–7.
Vieira A, Berry L, Ofosu F, Andrew M. Heparin sensitivity and resistance in the neonate: an explanation. Thromb Res. 1991;63:85–98.
Massicotte P, Adams M, Marzinotto V, Brooker LA, Andrew M. Low-molecular-weight heparin in pediatric patients with thrombotic disease: a dose finding study. J Pediatr. 1996;128:313–8.
Ignjatovic V, Najid S, Newall F, Summerhayes R, Monagle P. Dosing and monitoring of enoxaparin (low molecular weight heparin) therapy in children. Br J Haematol. 2010;149:734–8.
Van Driest SL, Choi L. Real-world data for pediatric pharmacometrics: can we upcycle clinical data for research use? Clin Pharmacol Ther. 2019;106:84–6.
Hornik CP, Atz AM, Bendel C, Chan F, Downes K, Grundmeier R, et al. Creation of a multicenter pediatric inpatient data repository derived from electronic health records. Appl Clin Inform. 2019;10:307–15.
Al-Sallami HS, Goulding A, Grant A, Taylor R, Holford N, Duffull SB. Prediction of fat-free mass in children. Clin Pharmacokinet. 2015;54:1169–78.
O’Hanlon CJ, Holford N, Sumpter A, Al-Sallami HS. Consistent methods for fat-free mass, creatinine clearance, and glomerular filtration rate to describe renal function from neonates to adults. CPT Pharmacometrics Syst Pharmacol. 2023;12:401–12.
Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics. 2012;130:1136–40.
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM 7.4 users guides [Internet]. 2022. Available from https://nonmem.iconplc.com/nonmem743/guides
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometr Syst Pharmacol. 2013;2:1–9.
Holford N, Heo Y-A, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–52.
Peters AM, Snelling HLR, Glass DM, Bird NJ. Estimation of lean body mass in children. Br J Anaesth. 2011;106:719–23.
Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Meibohm B, Läer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:475–87.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Gerhart JG, Carreño FO, Edginton AN, Sinha J, Perrin EM, Kumar KR, et al. Development and evaluation of a virtual population of children with obesity for physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2022;61:307–20.
Keizer RJ, Jansen RS, Rosing H, Thijssen B, Beijnen JH, Schellens JHM, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3:1–15.
Moffett BS, Lee-Kim YN, Galati M, Mahoney D, Shah MD, Teruya J, et al. Population pharmacokinetics of enoxaparin in pediatric patients. Ann Pharmacother. 2018;52:140–6.
Moffett BS, Galati M, Mahoney D, Lee-Kim YN, Teruya J, Shah MD, et al. Enoxaparin population pharmacokinetics in the first year of life. Ther Drug Monit. 2017;39:632–9.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67.
Ignjatovic V, Mertyn E, Monagle P. The coagulation system in children: developmental and pathophysiological considerations. Semin Thromb Hemost. 2011;37:723–9.
Derbalah A, Duffull S, Sherwin CM, Job K, Al-Sallami H. Optimal dosing of enoxaparin in overweight and obese children. Br J Clin Pharmacol. 2022;88:5348–58.
Gerhart JG, Carreño FO, Loop MS, Lee CR, Edginton AN, Sinha J, et al. Use of real-world data and physiologically-based pharmacokinetic modeling to characterize enoxaparin disposition in children with obesity. Clin Pharmacol Ther. 2022;112:391–403.
Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2003;56:96–103.
Barras M, Legg A. Drug dosing in obese adults. Aust Prescr. 2017;40:189–93.
Han PY, Duffull SB, Kirkpatrick CMJ, Green B. Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther. 2007;82:505–8.
Acknowledgments
Pediatric Trials Network (PTN) Steering Committee Members: Daniel K. Benjamin Jr., Kanecia Zimmerman, Phyllis Kennel, Cheryl Alderman, Zoe Sund, Kylie Opel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Scottsdale, AZ; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS; Leanne West, International Children’s Advocacy Network; Susan Abdel-Rahman. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Ravinder Anand, Elizabeth Payne, Lily Chen, Gina Simone, Kathleen O'Connor, Jennifer Cermak, and Lawrence Taylor, The Emmes Company, LLC (Data Coordinating Center). PTN Publications Committee: Thomas Green (Chair), Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Danny Benjamin; Perdita Taylor-Zapata; Kelly Wade; Greg Kearns; Ravinder Anand; Ian Paul; Julie Autmizguine; Edmund Capparelli; Kanecia Zimmerman; Rachel Greenberg; Cheryl Alderman; Terren Green. The PTN Data Repository sites principal investigator (study team) are as follows: Andrew Atz (Leslie Lenert, John Clark, and Kalyan Chundru), Medical University of South Carolina, Charleston, SC, USA; Catherine Bendel (Brian Harvey and Sonya Grillo), University of Minnesota, Minneapolis, MN, USA; Francis Chan (Stephanie Fan), Lorma Linda University, Lorma Linda, CA, USA; Kevin Downes and Robert Grundmeier (Mark Ramos and Shawn O’Connor, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; Benjamin Fogel, Penn State University, University Park, PA, USA; Debbie Gipson (Samara Attala, Richard Eickstadt, Erin Kaleba, Don Liamini, Jamie Estill, Jeremy Jared, and Peter Bow), University of Michigan, Ann Arbor, MI, USA; Matt Laughon (Jennifer Talbert and Cindy Clark), University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Michael Miller, William Muller, Ann and Robert H. Lurie Childrens Hospital of Chicago, Chicago, IL, USA; Michael Smith (Janice Sullivan, Steve Heilman, KP Singh, Satish Vuyyuri, Jeff Schwitters, and Don Stone), University of Louisville and Norton Healthcare, Louisville, KY, USA. We would like to acknowledge Terren Green for providing editorial support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under award 5R01HD096435. The enoxaparin pediatric data were collected by the Pediatric Trials Network (PTN) through NICHD contract HHSN275201000003I (Principal Investigator (PI): Daniel K. Benjamin, Jr.). F.O.C. and V.E.H. were supported through a University of North Carolina at Chapel Hill/GlaxoSmithKline (GSK) Pharmacokinetics/Pharmacodynamics Fellowship. J.G.G. received research support from a National Institute of General Medical Sciences (NIGMS) funded T32 program (T32GM122741), a Fred Eshelman Pre-Doctoral Fellowship in Pharmaceutical Sciences from the American Foundation for Pharmaceutical Education (AFPE), and a PharmAlliance travel award. D.G. received research support from the NICHD (5R01HD096435 and 1R01HD102949). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
V.E.H.’s affiliation with GSK did not present any conflict of interest regarding the research, findings, or conclusions presented in this manuscript. The other authors have no relevant conflicts of interest to disclose. The work conducted and reported herein is free from any financial or personal relationships that could potentially bias the results or interpretations.
Availability of Data and Materials
To help expand the knowledge base for pediatric medicine, the PTN is pleased to share data from its completed and published studies with interested investigators. For requests, please contact PTN-Program-Manager@dm.duke.edu.
Ethics Approval
The Institutional Review Board approved the protocol for this retrospective cohort study at Duke University (as the coordinating center) and all participating sites.
Consent to Participate
A waiver of informed consent was granted by the Institutional Review Board.
Consent for Publication
Not applicable.
Code Availability
NONMEM control stream code for the final model is available as Electronic Supplementary Material.
Author Contributions
Performed the research: F.O.C., J.G.G., J.S., K.K., C.H., and D.G. Participated in research design: F.O.C., J.G.G., J.S., K.K., C.H., and D.G. Performed the data analysis: F.O.C., J.G.G., and V.E.H. Wrote or contributed to the writing of the manuscript: F.O.C., J.G.G., V.E.H., J.S., K.K., C.K., C.H., and D.G.
Additional information
The Best Pharmaceuticals for Children Act–Pediatric Trials Network Steering Committee are listed in Acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carreño, F.O., Gerhart, J.G., Helfer, V.E. et al. Characterizing Enoxaparin’s Population Pharmacokinetics to Guide Dose Individualization in the Pediatric Population. Clin Pharmacokinet (2024). https://doi.org/10.1007/s40262-024-01388-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s40262-024-01388-x