Abstract
Background and Objective
The optimal choice for first- and second-line antiseizure medications for pediatric patients with convulsive status epilepticus remains ambiguous. The present study aimed to estimate the comparative effect on the efficacy and safety of different antiseizure medications in pediatric patients with status epilepticus and provide evidence for clinical practice.
Methods
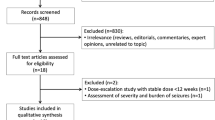
We searched PubMed, EMBASE, and the Cochrane Library for eligible randomized controlled trials. Inclusion criteria included: (1) pediatric patients; (2) diagnosis of status epilepticus; and (3) randomized controlled trials. Exclusion criteria were: (1) mixed population without a pediatric subgroup analysis; (2) not status epilepticus; (3) received the study drug prior to admission; (4) sample size fewer than 30; and (5) not randomized controlled trials. Primary outcome was seizure cessation. Secondary outcomes were seizure recurrence within 24 h, respiratory depression, and admission to an intensive care unit. The hierarchy of competing antiseizure medications was presented using the surface under the cumulative ranking curve.
Results
Eight first-line antiseizure medication studies involving 1686 participants and eight second-line antiseizure medication studies involving 1711 participants were eligible for analysis. Midazolam, diazepam, lorazepam, and paraldehyde were administered as first-line antiseizure medications. Valproate, phenobarbital, phenytoin, fosphenytoin, and levetiracetam were investigated as second-line antiseizure medications. No significant differences were observed across first- and second-line antiseizure medications. Midazolam ranked the best for primary and secondary outcomes among the first-line antiseizure medications. Phenobarbital ranked the best for seizure cessation and a lower risk of admission to the intensive care unit. Valproate had superiority in preventing recurrence within 24 h. Levetiracetam had the lowest probability of develo** respiratory depression.
Conclusions
This study demonstrated the hierarchy of competing interventions. Midazolam could be a better option for first-line treatment. Phenobarbital, levetiracetam, and valproate had their respective superiority in the second-line intervention. This study may provide useful information for clinical decision making under different circumstances.





Similar content being viewed by others
References
Gurcharran K, Grinspan ZM. The burden of pediatric status epilepticus: epidemiology, morbidity, mortality, and costs. Seizure. 2019;68:3–8.
Trinka E, Kalviainen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73.
Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61.
Trinka E, Brigo F, Shorvon S. Recent advances in status epilepticus. Curr Opin Neurol. 2016;29(2):189–98.
Beckett RD, Loeser KC, Bowman KR, Towne TG. Intention-to-treat and transparency of related practices in randomized, controlled trials of anti-infectives. BMC Med Res Methodol. 2016;16(1):106.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Lin L, Zhang J, Hodges JS, Chu H. Performing arm-based network meta-analysis in R with the pcnetmeta package. J Stat Softw. 2017;80:5.
Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–6.
Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet. 2006;367(9522):1591–7.
Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121(1):e58–64.
Sreenath TG, Gupta P, Sharma KK, Krishnamurthy S. Lorazepam versus diazepam-phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. Eur J Paediatr Neurol. 2010;14(2):162–8.
Malamiri RA, Ghaempanah M, Khosroshahi N, Nikkhah A, Bavarian B, Ashrafi MR. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. 2012;16(5):536–41.
Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311(16):1652–60.
Malu CKK, Kahamba DM, Walker TD, Mukampunga C, Musalu EM, Kokolomani J, et al. Efficacy of sublingual lorazepam versus intrarectal diazepam for prolonged convulsions in sub-Saharan Africa. J Child Neurol. 2014;29(7):895–902.
Momen AA, Azizi Malamiri R, Nikkhah A, Jafari M, Fayezi A, Riahi K, et al. Efficacy and safety of intramuscular midazolam versus rectal diazepam in controlling status epilepticus in children. Eur J Paediatr Neurol. 2015;19(2):149–54.
Welch RD, Nicholas K, Durkalski-Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population. Epilepsia. 2015;56(2):254–62.
Burman RJ, Ackermann S, Shapson-Coe A, Ndondo A, Buys H, Wilmshurst JM. A comparison of parenteral phenobarbital vs. parenteral phenytoin as second-line management for pediatric convulsive status epilepticus in a resource-limited setting. Front Neurol. 2019;10:506.
Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019;393(10186):2135–45.
Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125–34.
Noureen N, Khan S, Khursheed A, Iqbal I, Maryam M, Sharib SM, et al. Clinical efficacy and safety of injectable levetiracetam versus phenytoin as second-line therapy in the management of generalized convulsive status epilepticus in children: an open-label randomized controlled trial. J Clin Neurol. 2019;15(4):468–72.
Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395(10231):1217–24.
Nalisetty S, Kandasamy S, Sridharan B, Vijayakumar V, Sangaralingam T, Krishnamoorthi N. Clinical effectiveness of levetiracetam compared to fosphenytoin in the treatment of benzodiazepine refractory convulsive status epilepticus. Indian J Pediatr. 2020;87:512–9.
Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020;57(3):222–7.
Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 2004;25(4):193–200.
Egunsola O, Choonara I, Sammons HM. Safety of levetiracetam in paediatrics: a systematic review. PLoS ONE. 2016;11(3):e0149686.
Li L, Zhang Y, Jia L, Jia D, Faramand A, Chong W, et al. Levetiracetam versus phenytoin for the treatment of established status epilepticus: a systematic review and meta-analysis of randomized controlled trials. Seizure. 2020;78:43–8.
Verrotti A, Prezioso G, Di Sabatino F, Franco V, Chiarelli F, Zaccara G. The adverse event profile of levetiracetam: a meta-analysis on children and adults. Seizure. 2015;31:49–55.
Stephen LJ, Brodie MJ. Brivaracetam: a novel antiepileptic drug for focal-onset seizures. Ther Adv Neurol Disord. 2018;11:1756285617742081.
Brigo F, Bragazzi NL, Nardone R, Trinka E. Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: results of an indirect comparison meta-analysis of RCTs. Seizure. 2016;42:29–37.
Brigo F, Lattanzi S, Nardone R, Trinka E. Intravenous brivaracetam in the treatment of status epilepticus: a systematic review. CNS Drugs. 2019;33(8):771–81.
Steinhoff BJ. The AMPA receptor antagonist perampanel in the adjunctive treatment of partial-onset seizures: clinical trial evidence and experience. Ther Adv Neurol Disord. 2015;8(3):137–47.
Brigo F, Lattanzi S, Rohracher A, Russo E, Meletti S, Grillo E, et al. Perampanel in the treatment of status epilepticus: a systematic review of the literature. Epilepsy Behav. 2018;86:179–86.
Kim HD, Chi CS, Desudchit T, Nikanorova M, Visudtibhan A, Nabangchang C, et al. Review of clinical studies of perampanel in adolescent patients. Brain Behav. 2016;6(9):e00505.
Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S. Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav. 2019;101(Pt B):106466.
Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S. Second-line treatments in benzodiazepine-resistant convulsive status epilepticus: an updated network meta-analysis including the ESET trial: what did change? Epilepsy Behav. 2020;106:107035.
Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial: survey of 71 "negative" trials. N Engl J Med. 1978;299(13):690–4.
Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol. 2010;67(8):931–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Yihao Zhang, Yingjie Liu, Qiao Liao, and Zhixiong Liu have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
The data used in this study were fully available in databases.
Code availability
Not applicable.
Author contributions
YZ and YL equally contributed to this manuscript in conception, data extraction, quality assessment, data analysis, and drafting. QL and ZL revised the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, Y., Liao, Q. et al. Preferential Antiseizure Medications in Pediatric Patients with Convulsive Status Epilepticus: A Systematic Review and Network Meta-Analysis. Clin Drug Investig 41, 1–17 (2021). https://doi.org/10.1007/s40261-020-00975-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00975-7