Abstract
Background and Objective
Deep brain stimulation (DBS) is an established treatment for Parkinson’s disease (PD) in patients with advanced motor symptoms with an inadequate response to pharmacotherapies. Despite its effectiveness, the cost effectiveness of DBS remains a subject of debate. This systematic review aims to update and synthesize evidence on the cost effectiveness of DBS for PD.
Methods
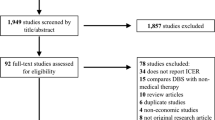
To identify full economic evaluations that compared the cost effectiveness of DBS with other best medical treatments, a comprehensive search was conducted of the PubMed, Embase, Scopus, and Tufts Cost-Effective Analysis registry databases. The selected papers were systematically reviewed, and the results were summarized. For the quality appraisal, we used the modified economic evaluations bias checklist. The review protocol was a priori registered with PROSPERO, CRD42022345508.
Results
Sixteen identified cost-utility analyses that reported 19 comparisons on the use of DBS for PD were systematically reviewed. The studies were primarily conducted in high-income countries and employed Markov models. The costs considered were direct costs: surgical expenses, calibration, pulse generator replacement, and annual drug expenses. The majority of studies used country-specific thresholds. Fourteen comparisons from 12 studies reported on the cost effectiveness of DBS compared to best medical treatments. Eleven comparisons reported DBS as cost effective based on incremental cost-utility ratio results.
Conclusions
The cost effectiveness of DBS for PD varies by time horizon, costs considered, threshold utilized, and stage of PD progression. Standardizing approaches and comparing DBS with other treatments are needed for future research on effective PD management.


Similar content being viewed by others
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease Nat Rev Dis Primers. 2017;3(1):17013.
Smith ER, Perrin PB, Tyler CM, Lageman SK, Villasenor T. Parkinson’s symptoms and caregiver burden and mental health: a cross-cultural mediational model. Behav Neurol. 2019;2019:1396572.
Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson’s disease. Trends Neurosci. 2000;23(10 Suppl.):S2-7.
National Collaborating Centre for Chronic Conditions (UK). Symptomatic pharmacological therapy in Parkinson’s disease. London: Royal College of Physicians; 2006.
Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson's disease: surgical technique and perioperative management. Mov Disord. 2006;21 Suppl. 14(S14):S247–58.
Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25(5):578–86.
Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013;123(11):4546–56.
National Institute for Health and Care Excellence. Parkinson's disease in adults. NICE guideline [NG71]. 2017. National Institute for Health and Care Excellence (NICE), London.
Rughani A, Schwalb JM, Sidiropoulos C, Pilitsis J, Ramirez-Zamora A, Sweet JA, et al. Congress of Neurological Surgeons systematic review and evidence-based guideline on subthalamic nucleus and globus pallidus internus deep brain stimulation for the treatment of patients with Parkinson’s disease: executive summary. Neurosurgery. 2018;82(6):753–6.
Nijhuis FAP, Esselink R, de Bie RMA, Groenewoud H, Bloem BR, Post B, et al. Translating evidence to advanced Parkinson’s disease patients: a systematic review and meta-analysis. Mov Disord. 2021;36(6):1293–307.
Perestelo-Perez L, Rivero-Santana A, Perez-Ramos J, Serrano-Perez P, Panetta J, Hilarion P. Deep brain stimulation in Parkinson’s disease: meta-analysis of randomized controlled trials. J Neurol. 2014;261(11):2051–60.
Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21 Suppl. 14(S14):S290–304.
Mansouri A, Taslimi S, Badhiwala JH, Witiw CD, Nassiri F, Odekerken VJJ, et al. Deep brain stimulation for Parkinson’s disease: meta-analysis of results of randomized trials at varying lengths of follow-up. J Neurosurg. 2018;128(4):1199–213.
Peng L, Fu J, Ming Y, Zeng S, He H, Chen L. The long-term efficacy of STN vs GPi deep brain stimulation for Parkinson disease: a meta-analysis. Medicine (Baltimore). 2018;97(35): e12153.
Zhang J, Li J, Chen F, Liu X, Jiang C, Hu X, et al. STN versus GPi deep brain stimulation for dyskinesia improvement in advanced Parkinson’s disease: a meta-analysis of randomized controlled trials. Clin Neurol Neurosurg. 2021;201: 106450.
Hacker ML, Currie AD, Molinari AL, Turchan M, Millan SM, Heusinkveld LE, et al. Subthalamic nucleus deep brain stimulation may reduce medication costs in early stage Parkinson’s disease. J Parkinsons Dis. 2016;6(1):125–31.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–60.
Becerra JE, Zorro O, Ruiz-Gaviria R, Castaneda-Cardona C, Otalora-Esteban M, Henao S, et al. Economic analysis of deep brain stimulation in Parkinson disease: systematic review of the literature. World Neurosurg. 2016;93:44–9.
Dams J, Balzer-Geldsetzer M, Siebert U, Deuschl G, Schuepbach WM, Krack P, et al. Cost-effectiveness of neurostimulation in Parkinson’s disease with early motor complications. Mov Disord. 2016;31(8):1183–91.
Dams J, Siebert U, Bornschein B, Volkmann J, Deuschl G, Oertel WH, et al. Cost-effectiveness of deep brain stimulation in patients with Parkinson’s disease. Mov Disord. 2013;28(6):763–71.
Eggington S, Valldeoriola F, Chaudhuri KR, Ashkan K, Annoni E, Deuschl G. The cost-effectiveness of deep brain stimulation in combination with best medical therapy, versus best medical therapy alone, in advanced Parkinson’s disease. J Neurol. 2014;261(1):106–16.
Fundament T, Eldridge PR, Green AL, Whone AL, Taylor RS, Williams AC, et al. Deep brain stimulation for Parkinson’s disease with early motor complications: a UK cost-effectiveness analysis. PLoS ONE. 2016;11(7): e0159340.
McIntosh E, Gray A, Daniels J, Gill S, Ives N, Jenkinson C, et al. Cost-utility analysis of deep brain stimulation surgery plus best medical therapy versus best medical therapy in patients with Parkinson’s: economic evaluation alongside the PD SURG trial. Mov Disord. 2016;31(8):1173–82.
Dang TTH, Rowell D, Connelly LB. Cost-effectiveness of deep brain stimulation with movement disorders: a systematic review. Move Disord Clin Pract. 2019;6(5):348–58.
Afentou N, Jarl J, Gerdtham UG, Saha S. Economic evaluation of interventions in Parkinson’s disease: a systematic literature review. Move Disord Clin Pract. 2019;6(4):282–90.
Marsili L, Bologna M, Miyasaki JM, Colosimo C. Parkinson’s disease advanced therapies-a systematic review: more unanswered questions than guidance. Parkinsonism Relat Disord. 2021;83:132–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
CEA Registry. Center for the Evaluation of Value and Risk in Health. https://cevr.tuftsmedicalcenter.org/databases/cea-registry. Accessed May 2021.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Ankit R. WebPlotDigitizer. 2021. https://automeris.io/WebPlotDigitizer. Accessed 12 Nov 2023.
Paulden M. Why it’s time to abandon the ICER. Pharmacoeconomics. 2020;38(8):781–4.
International Monetary Fund. World Economic Outlook database, April 2023. 2023. https://www.imf.org/en/Publications/WEO/weo-database/2023/April/download-entire-database. Accessed 12 Nov 2023.
Microsoft Corporation. Microsoft Excel. 2018. https://office.microsoft.com/excel. Accessed 12 Nov 2023.
StataCorp. Stata statistical software: release 16. 2019;16. https://www.stata.com/. Accessed 12 Nov 2023.
Adarkwah CC, van Gils PF, Hiligsmann M, Evers SM. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):513–23.
Horstink M, Tolosa E, Bonuccelli U, Deuschl G, Friedman A, Kanovsky P, et al. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson's disease. Eur J Neurol. 2006;13(11):1186–202.
Walter E, Odin P. Cost-effectiveness of continuous subcutaneous apomorphine in the treatment of Parkinson’s disease in the UK and Germany. J Med Econ. 2015;18(2):155–65.
Valldeoriola F, Morsi O, Tolosa E, Rumià J, Martí MJ, Martínez-Martín P. Prospective comparative study on cost-effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson’s disease. Mov Disord. 2007;22(15):2183–91.
Vivancos-Matellano F, Garcia-Ruiz A, Garcia-Agua SN. Pharmacoeconomic study of the treatment of advanced Parkinson’s disease. Rev Neurol. 2016;63(12):529–36.
Pietzsch JB, Garner AM, Marks WJJ. Cost-effectiveness of deep brain stimulation for advanced Parkinson’s disease in the United States. Neuromodulation. 2016;19(7):689–97.
Fann JC, Chang KC, Yen AM, Chen SL, Chiu SY, Chen HH, et al. Cost-effectiveness analysis of deep brain stimulation for Parkinson disease in Taiwan. World Neurosurg. 2020;138:e459–68.
Norlin JM, Willis M, Persson U, Andersson E, Pålhagen E, Odin P. Swedish guidelines for device-aided therapies in Parkinson’s disease: economic evaluation and implementation. Acta Neurol Scand. 2021;144(2):170–8.
Tomaszewski KJ, Holloway RG. Deep brain stimulation in the treatment of Parkinson’s disease: a cost-effectiveness analysis. Neurology. 2001;57(4):663–71.
Mahajan UV, Ravikumar VK, Kumar KK, Ku S, Ojukwu DI, Kilbane C, et al. Bilateral deep brain stimulation is the procedure to beat for advanced Parkinson disease: a meta-analytic, cost-effective threshold analysis for focused ultrasound. Neurosurgery. 2021;88(3):487–96.
Zhu XL, Chan DT, Lau CK, Poon WS, Mok VC, Chan AY, et al. Cost-effectiveness of subthalmic nucleus deep brain stimulation for the treatment of advanced Parkinson disease in Hong Kong: a prospective study. World Neurosurg. 2014;82(6):987–93.
Kawamoto Y, Mouri M, Taira T, Iseki H, Masamune K. Cost-effectiveness analysis of deep brain stimulation in patients with Parkinson’s disease in Japan. World Neurosurg. 2016;89:628-35.e1.
Meng Y, Pople CB, Kalia SK, Kalia L, Davidson B, Bigioni L, et al. Cost-effectiveness analysis of MR-guided focused ultrasound thalamotomy for tremor-dominant Parkinson’s disease. J Neurosurg. 2020;136(1):273–8.
Young MK, Ng SK, Mellick G, Scuffham PA. Map** of the PDQ-39 to EQ-5D scores in patients with Parkinson’s disease. Qual Life Res. 2013;22(5):1065–72.
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–22.
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908.
Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9(6):581–91.
Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68(2):165.
Fasano A, Antonini A, Katzenschlager R, Krack P, Odin P, Evans AH, et al. Management of advanced therapies in Parkinson’s disease patients in times of humanitarian crisis: the COVID-19 experience. Mov Disord Clin Pract. 2020;7(4):361–72.
Bagepally BS, Chaikledkaew U, Chaiyakunapruk N, Attia J, Thakkinstian A. Meta-analysis of economic evaluation studies: data harmonisation and methodological issues. BMC Health Serv Res. 2022;22(1):202.
Crespo C, Monleon A, Díaz W, Ríos M. Comparative efficiency research (COMER): meta-analysis of cost-effectiveness studies. BMC Med Res Methodol. 2014;22(14):139.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Department of Health Research, Government of India funds the HTARC, ICMR-NIE grant no. T.11011/08/2017-HR(Part-1)/E-office-8025571 dated 27 November, 2019. The funders played no role in the study’s conception, execution, or manuscript preparation.
Conflict of interest
Akhil Sasidharan, Bhavani Shankara Bagepally, and S. Sajith Kumar have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All relevant data and materials supporting the findings of this study are included in the submitted files. Additional data or materials, if required, can be obtained upon reasonable request by contacting the corresponding author.
Code availability
Not applicable.
Author contributions
BSB and AS designed the study. BSB, AS, and SK conducted the literature search, screening, and data extraction. AS and SK performed the quality assessment, and AS performed the data synthesis. AS drafted the manuscript. All authors contributed to the interpretation of the results and critically revised the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sasidharan, A., Bagepally, B.S. & Kumar, S. Cost Effectiveness of Deep Brain Stimulation for Parkinson’s Disease: A Systematic Review. Appl Health Econ Health Policy 22, 181–192 (2024). https://doi.org/10.1007/s40258-023-00848-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00848-y