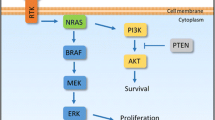
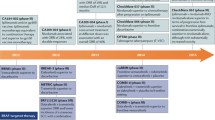
Abstract
There have been many recent advances in melanoma therapy. While 50% of melanomas have a BRAF mutation and are a target for BRAF inhibitors, the remaining 50% are BRAF wild-type. Immune checkpoint inhibitors targeting PD-1, cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and lymphocyte activated gene-3 (Lag-3) are all approved for the treatment of patients with advanced BRAF wild-type melanoma; however, treatment of this patient population following initial immune checkpoint blockade is a current therapeutic challenge given the lack of other efficacious options. Here, we briefly review available US FDA-approved therapies for BRAF wild-type melanoma and focus on develo** treatment avenues for this heterogeneous group of patients. We review the basics of genomic features of both BRAF mutant and BRAF wild-type melanoma as well as efforts underway to develop new targeted therapies involving the mitogen-activated protein kinase (MAPK) pathway for patients with BRAF wild-type tumors. We then focus on novel immunotherapies, including develo** checkpoint inhibitors and agonists, cytokine therapies, oncolytic viruses and tumor-infiltrating lymphocytes, all of which represent potential therapeutic avenues for patients with BRAF wild-type melanoma who progress on currently approved immune checkpoint inhibitors.


Similar content being viewed by others
References
Ribas A, Wolchok JD. Combining cancer immunotherapy and targeted therapy. Curr Opin Immunol. 2013;25(2):291–6. https://doi.org/10.1016/j.coi.2013.02.011.
Allison JP, Krummel MF. The Yin and Yang of T cell costimulation. Science. 1995;270(5238):932–3. https://doi.org/10.1126/science.270.5238.932.
Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–72. https://doi.org/10.1093/intimm/8.5.765.
Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. https://doi.org/10.1084/jem.192.7.1027.
Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291(1):1–13. https://doi.org/10.1111/nyas.12180.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/NEJMoa1003466.
Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. https://doi.org/10.1016/s1470-2045(09)70334-1.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. https://doi.org/10.1056/NEJMoa1305133.
Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in Patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–12. https://doi.org/10.1200/jco.2014.58.3708.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. https://doi.org/10.1056/NEJMoa1503093.
Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–90. https://doi.org/10.1200/jco.2016.71.8023.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. https://doi.org/10.1056/NEJMoa1504030.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–56. https://doi.org/10.1056/NEJMoa1709684.
Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. https://doi.org/10.1056/NEJMoa2109970.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. https://doi.org/10.1038/nature00766.
Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16(6):345–58. https://doi.org/10.1038/nrc.2016.37.
Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–71. https://doi.org/10.1146/annurev-pathol-012513-104658.
Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer. 2014;14(7):455–67. https://doi.org/10.1038/nrc3760.
Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol. 2012;30(20):2522–9. https://doi.org/10.1200/jco.2011.41.2452.
Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Investig. 2017;97(2):146–57. https://doi.org/10.1038/labinvest.2016.142.
Rabbie R, Ferguson P, Molina-Aguilar C, Adams DJ, Robles-Espinoza CD. Melanoma subtypes: genomic profiles, prognostic molecular markers and therapeutic possibilities. J Pathol. 2019;247(5):539–51. https://doi.org/10.1002/path.5213.
Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–80. https://doi.org/10.1038/nature22071.
Newell F, Johansson PA, Wilmott JS, et al. Comparative genomics provides etiologic and biological insight into melanoma subtypes. Cancer Discov. 2022;12(12):2856–79. https://doi.org/10.1158/2159-8290.Cd-22-0603.
Yeh I, Jorgenson E, Shen L, et al. Targeted genomic profiling of acral melanoma. J Natl Cancer Inst. 2019;111(10):1068–77. https://doi.org/10.1093/jnci/djz005.
Pham DDM, Guhan S, Tsao H. KIT and melanoma: biological insights and clinical implications. Yonsei Med J. 2020;61(7):562–71. https://doi.org/10.3349/ymj.2020.61.7.562.
Yeh I, Bastian BC. Melanoma pathology: new approaches and classification. Br J Dermatol. 2021;185(2):282–93. https://doi.org/10.1111/bjd.20427.
Moran JMT, Le LP, Nardi V, et al. Identification of fusions with potential clinical significance in melanoma. Mod Pathol. 2022;35(12):1837–47. https://doi.org/10.1038/s41379-022-01138-z.
Yen I, Shanahan F, Lee J, et al. ARAF mutations confer resistance to the RAF inhibitor belvarafenib in melanoma. Nature. 2021;594(7863):418–23. https://doi.org/10.1038/s41586-021-03515-1.
de Braud F, Dooms C, Heist RS, et al. Initial evidence for the efficacy of naporafenib in combination with trametinib in NRAS-mutant melanoma: results from the expansion arm of a phase Ib, open-label study. J Clin Oncol. 2023;41(14):2651–60. https://doi.org/10.1200/jco.22.02018.
Lebbe C. LBA40 Phase II study of multiple LXH254 drug combinations in patients (pts) with unresectable/metastatic, BRAF V600- or NRAS-mutant melanoma. Ann Oncol. 2022;33(7):S1407.
Sullivan RJ, Infante JR, Janku F, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8(2):184–95. https://doi.org/10.1158/2159-8290.Cd-17-1119.
Tarin M, Némati F, Decaudin D, et al. FAK inhibitor-based combinations with MEK or PKC inhibitors trigger synergistic antitumor effects in uveal melanoma. Cancers. 2023. https://doi.org/10.3390/cancers15082280.
Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016;45:129–38. https://doi.org/10.1016/j.ctrv.2016.03.002.
Steeb T, Wessely A, Petzold A, et al. c-Kit inhibitors for unresectable or metastatic mucosal, acral or chronically sun-damaged melanoma: a systematic review and one-arm meta-analysis. Eur J Cancer. 2021;157:348–57. https://doi.org/10.1016/j.ejca.2021.08.015.
Kim KB, Eton O, Davis DW, et al. Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br J Cancer. 2008;99(5):734–40. https://doi.org/10.1038/sj.bjc.6604482.
Arance A, de la Cruz-Merino L, Petrella TM, et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. 2023;41(1):75–85. https://doi.org/10.1200/jco.22.00221.
Algazi AP, Esteve-Puig R, Nosrati A, et al. Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma. Pigment Cell Melanoma Res. 2018;31(1):110–4. https://doi.org/10.1111/pcmr.12644.
Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50. https://doi.org/10.1038/s41591-018-0014-x.
Iijima N, Iwasaki A. Tissue instruction for migration and retention of TRM cells. Trends Immunol. 2015;36(9):556–64. https://doi.org/10.1016/j.it.2015.07.002.
Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–52. https://doi.org/10.1016/j.ccell.2014.09.007.
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–65. https://doi.org/10.1084/jem.182.2.459.
Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5950–6. https://doi.org/10.1200/jco.2008.16.1927.
Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18(2):206–13. https://doi.org/10.1016/j.coi.2006.01.011.
Olson DJ, Eroglu Z, Brockstein B, et al. Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol. 2021;39(24):2647–55. https://doi.org/10.1200/jco.21.00079.
Graydon CG, Mohideen S, Fowke KR. LAG3’s enigmatic mechanism of action. Front Immunol. 2020;11: 615317. https://doi.org/10.3389/fimmu.2020.615317.
Ascierto PA, Lipson EJ, Dummer R, et al. Nivolumab and relatlimab in patients with advanced melanoma that had progressed on anti-programmed death-1/programmed death ligand 1 therapy: results from the phase I/IIa RELATIVITY-020 trial. J Clin Oncol. 2023;41(15):2724–35. https://doi.org/10.1200/jco.22.02072.
Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682–5. https://doi.org/10.1038/nm0697-682.
Gauttier V, Judor JP, Le Guen V, Cany J, Ferry N, Conchon S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer. 2014;135(12):2857–67. https://doi.org/10.1002/ijc.28943.
Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186(1):47–55. https://doi.org/10.1084/jem.186.1.47.
Menk AV, Schar** NE, Rivadeneira DB, et al. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med. 2018;215(4):1091–100. https://doi.org/10.1084/jem.20171068.
Kawalekar OU, O’Connor RS, Fraietta JA, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380–90. https://doi.org/10.1016/j.immuni.2016.01.021.
Segal NH, Logan TF, Hodi FS, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2017;23(8):1929–36. https://doi.org/10.1158/1078-0432.Ccr-16-1272.
Segal NH, He AR, Doi T, et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin Cancer Res. 2018;24(8):1816–23. https://doi.org/10.1158/1078-0432.Ccr-17-1922.
Chen S, Lee LF, Fisher TS, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3(2):149–60. https://doi.org/10.1158/2326-6066.Cir-14-0118.
Shindo Y, Yoshimura K, Kuramasu A, et al. Combination immunotherapy with 4-1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res. 2015;35(1):129–36.
Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47–58. https://doi.org/10.1146/annurev-med-062518-045435.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–85. https://doi.org/10.1038/s41577-019-0224-6.
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–94. https://doi.org/10.1084/jem.20100643.
Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–21. https://doi.org/10.1038/nature19330.
Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. https://doi.org/10.1111/imr.12520.
Hamid O, Mehmi I, Dudzisz-Sledz M, Hoyle PE, Wei W, Powderly JD. A phase 1/2 study of retifanlimab (INCMGA00012, anti-PD-1), INCAGN02385 (anti-LAG-3), and INCAGN02390 (anti–TIM-3) combination therapy in patients (Pts) with advanced solid tumors. J Clin Oncol. 2023;41(16 suppl):2599–2599.
Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–37. https://doi.org/10.1016/j.ccell.2014.10.018.
Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–32. https://doi.org/10.1038/s41590-018-0132-0.
Wang Y, Du J, Gao Z, et al. Evolving landscape of PD-L2: bring new light to checkpoint immunotherapy. Br J Cancer. 2023;128(7):1196–207. https://doi.org/10.1038/s41416-022-02084-y.
Demerlé C, Gorvel L, Olive D. BTLA-HVEM couple in health and diseases: insights for immunotherapy in lung cancer. Front Oncol. 2021;11: 682007. https://doi.org/10.3389/fonc.2021.682007.
Dong P, **ong Y, Yue J, Hanley SJB, Watari H. B7H3 as a promoter of metastasis and promising therapeutic target. Front Oncol. 2018;8:264. https://doi.org/10.3389/fonc.2018.00264.
Buchan SL, Rogel A, Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood. 2018;131(1):39–48. https://doi.org/10.1182/blood-2017-07-741025.
Amatore F, Gorvel L, Olive D. Role of inducible co-stimulator (ICOS) in cancer immunotherapy. Expert Opin Biol Ther. 2020;20(2):141–50. https://doi.org/10.1080/14712598.2020.1693540.
Zappasodi R, Sirard C, Li Y, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25(5):759–66. https://doi.org/10.1038/s41591-019-0420-8.
Gu SS, Zhang W, Wang X, et al. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov. 2021;11(6):1524–41. https://doi.org/10.1158/2159-8290.Cd-20-0812.
Chen X, Lu Q, Zhou H, et al. A membrane-associated MHC-I inhibitory axis for cancer immune evasion. Cell. 2023;186(18):3903-3920.e21. https://doi.org/10.1016/j.cell.2023.07.016.
Bentebibel SE, Diab A. Cytokines in the treatment of melanoma. Curr Oncol Rep. 2021;23(7):83. https://doi.org/10.1007/s11912-021-01064-4.
Diab A, Gogas H, Sandhu S, et al. Bempegaldesleukin plus nivolumab in untreated advanced melanoma: the open-label, phase III PIVOT IO 001 trial results. J Clin Oncol. 2023. https://doi.org/10.1200/jco.23.00172.
Greaney SK, Algazi AP, Tsai KK, et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol Res. 2020;8(2):246–54. https://doi.org/10.1158/2326-6066.Cir-19-0359.
Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18(8):1614–21. https://doi.org/10.1200/jco.2000.18.8.1614.
Lawson DH, Lee S, Zhao F, et al. Randomized, placebo-controlled, phase III trial of yeast-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) versus peptide vaccination versus GM-CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: a trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). J Clin Oncol. 2015;33(34):4066–76. https://doi.org/10.1200/jco.2015.62.0500.
Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7(1):145. https://doi.org/10.1186/s40425-019-0623-z.
Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109-1119.e10. https://doi.org/10.1016/j.cell.2017.08.027.
Andtbacka RHI, Curti B, Daniels GA, et al. Clinical responses of oncolytic coxsackievirus A21 (V937) in patients with unresectable melanoma. J Clin Oncol. 2021;39(34):3829–38. https://doi.org/10.1200/jco.20.03246.
Chmielowski B, Milhem MM, Sacco JJ, et al. Initial efficacy and safety of RP1 + nivolumab in patients with anti-PD-1-failed melanoma from the ongoing phase 1/2 IGNYTE study. J Clin Oncol. 2023;41(16 suppl):9509–9509. https://doi.org/10.1200/JCO.2023.41.16_suppl.9509.
Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. https://doi.org/10.1158/1078-0432.Ccr-11-0116.
Ellebaek E, Iversen TZ, Junker N, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:169. https://doi.org/10.1186/1479-5876-10-169.
Sarnaik AA, Hamid O, Khushalani NI, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol. 2021;39(24):2656–66. https://doi.org/10.1200/jco.21.00612.
Chesney J, Lewis KD, Kluger H, et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2022-005755.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding from the Cook/Juelsgaard and Amoroso Foundation (AD).
Conflict of Interest
AH declares no conflicts of interest. AD: stock and other ownership interests—Neuvogen, Trex bio. Honoraria—EMD Serono, Inovio Pharmaceuticals. Consulting or advisory role—Genoptix, GlaxoSmithKline, Oncosec, Caris, Eisai, GLG. Speakers' bureau—no relationships to disclose. Research Funding—Bristol-Myers Squibb, Checkmate Pharmaceuticals, Genentech/Roche (Inst), GlaxoSmithKline (Inst), Incyte, Merck/Schering Plough (Inst), Novartis, Oncosec (Inst), Pfizer (Inst).
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
No datasets were generated or analyzed during the current study.
Code Availability
No code was generated.
Author Contributions
Planning and conception AH, AD; writing AH, AD; final editing AH, AD.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haugh, A., Daud, A.I. Therapeutic Strategies in BRAF V600 Wild-Type Cutaneous Melanoma. Am J Clin Dermatol 25, 407–419 (2024). https://doi.org/10.1007/s40257-023-00841-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-023-00841-0