Abstract
Objectives
Diabetic neuropathic pain (DNP) is a debilitating symptom of diabetic neuropathy which seriously impairs patient’s quality of life. Currently, there is no specific therapy for DNP except for duloxetine and gabapentin that show limited utility in alleviating DNP. The present review aims to discuss the central role of protein kinases in the pathogenesis of DNP and their therapeutic modulation.
Methods
Scopus, PubMed, and Google scholar were searched up to January 2022 to find relevant studies with English language in which the roles of proteins kinases in DNP were examined.
Results
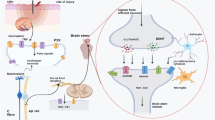
DNP is associated with hyperactivity in pain sensory neurons and therapies aim to specifically suppress redundant discharges in these neurons without affecting the activity of other sensory and motor neurons. Transient receptor potential vanilloid 1 (TRPV1) and purinergic 2 × 7 receptors (P2 × 7R) are two receptor channels, highly expressed in pain sensory neurons and their blockade produces remarkable analgesic effects in DNP. The activities of receptor channels are mainly regulated by the protein kinases whose modulation provides remarkable analgesic effects in DNP models.
Conclusion
Capsaicin, TRPV1 modulator, is the only agent successfully examined in clinical trials with promising effects in patients with DNP. Current data suggest that blocking calcium calmodulin dependent protein kinase II (CaMKII) is superior to other approaches, considering its pivotal role in regulating the pain neuron potentials. By this means, DNP alleviation is achievable without affecting the activity of other sensory or motor neurons.

Similar content being viewed by others
Data Availability
This article does not contain any studies with human participants or animals performed by any of the authors.
References
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat reviews Disease primers. 2019;5:1–18.
Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21:922–36.
Gylfadottir SS, Itani M, Kristensen AG, Karlsson P, Krøigård T, Bennett DL, et al. The characteristics of pain and dysesthesia in patients with diabetic polyneuropathy. PLoS ONE. 2022;17:e0263831.
Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36:2456–65.
Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–22.
Feldman EL, Nave K-A, Jensen TS, Bennett DL. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–313.
Gupta M, Knezevic NN, Abd-Elsayed A, Ray M, Patel K, Chowdhury B. Treatment of painful diabetic neuropathy—a narrative review of pharmacological and interventional approaches. Biomedicines. 2021;9:573.
Graham RD, Bruns TM, Duan B, Lempka SF. Dorsal root ganglion stimulation for chronic pain modulates Aβ-fiber activity but not C-fiber activity: a computational modeling study. Clin Neurophysiol. 2019;130:941–51.
Roskoski R Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol Res. 2015;100:1–23.
Ardito F, Giuliani M, Perrone D, Troiano G, Lo Muzio L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int J Mol Med. 2017;40:271–80.
Paoletti P, Ellis-Davies GC, Mourot A. Optical control of neuronal ion channels and receptors. Nat Rev Neurosci. 2019;20:514–32.
Woll KA, Van Petegem F. Calcium-release channels: structure and function of IP3 receptors and ryanodine receptors. Physiol Rev. 2022;102:209–68.
Duran C, Thompson CH, **ao Q, Hartzell HC. Chloride channels: often enigmatic, rarely predictable. Annu Rev Physiol. 2010;72:95–121.
Fallah HP, Ahuja E, Lin H, Qi J, He Q, Gao S, et al. A review on the role of TRP channels and their potential as drug Targets_An Insight into the TRP Channel Drug Discovery Methodologies. Front Pharmacol. 2022;13:1784.
Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10.
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–7.
Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S-i. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol. 2001;422:83–6.
Forst T, Pohlmann T, Kunt T, Goitom K, Schulz G, Löbig M, et al. The influence of local capsaicin treatment on small nerve fibre function and neurovascular control in symptomatic diabetic neuropathy. Acta Diabetol. 2002;39:1–6.
Vinik AI, Perrot S, Vinik EJ, Pazdera L, Jacobs H, Stoker M, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16:1–14.
Simpson DM, Robinson-Papp J, Van J, Stoker M, Jacobs H, Snijder RJ, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42–53.
Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, et al. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62.
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29.
Reid G, Flonta M-L. Ion channels activated by cold and menthol in cultured rat dorsal root ganglion neurones. Neurosci Lett. 2002;324:164–8.
Liu B, Qin F. Functional control of cold-and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4, 5-bisphosphate. J Neurosci. 2005;25:1674–81.
Pabbidi MR, Premkumar LS. Role of transient receptor potential channels Trpv1 and Trpm8 in diabetic peripheral neuropathy. Journal of diabetes and treatment 2017; 2017.
Duan B, Wu L-J, Yu Y-Q, Ding Y, **g L, Xu L, et al. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27:11139–48.
Bektur E, Şahin E, Ceyhan E, Donmez DB, Canbek M, Baycu C, et al. Beneficial effect of mirtazapine on diabetes-induced hyperalgesia: involvement of TRPV1 and ASIC1 channels in the spinal cord and dorsal root ganglion. Neurol Res. 2019;41:544–53.
Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–53.
**e Y-K, Luo H, Zhang S-X, Chen X-Y, Guo R, Qiu X-Y, et al. GPR177 in A-fiber sensory neurons drives diabetic neuropathic pain via WNT-mediated TRPV1 activation. Sci Transl Med. 2022;14:eabh2557.
Wei L, Caseley E, Li D, Jiang L-H. ATP-induced P2X receptor-dependent large pore formation: how much do we know? Frontiers Media SA; 2016. p. 5.
Janks L, Sharma CV, Egan TM. A central role for P2X7 receptors in human microglia. J Neuroinflamm. 2018;15:1–18.
Wang A, Shi X, Yu R, Qiao B, Yang R, Xu C. The P2X7 Receptor Is Involved in Diabetic Neuropathic Pain Hypersensitivity Mediated by TRPV1 in the Rat Dorsal Root Ganglion.Frontiers in Molecular Neuroscience2021:104.
Chen L, Wang H, **ng J, Shi X, Huang H, Huang J, et al. Silencing P2X7R alleviates Diabetic Neuropathic Pain Involving TRPV1 via PKCε/P38MAPK/NF-κB signaling pathway in rats. Int J Mol Sci. 2022;23:14141.
Wilkerson JL, Alberti LB, Kerwin AA, Ledent CA, Thakur GA, Makriyannis A, et al. Peripheral versus central mechanisms of the cannabinoid type 2 receptor agonist AM1710 in a mouse model of neuropathic pain. Brain and behavior. 2020;10:e01850.
Kao D-J, Li AH, Chen J-C, Luo R-S, Chen Y-L, Lu J-C, et al. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1. 8 sodium channels in dorsal root ganglion neurons. J Neuroinflamm. 2012;9:1–13.
Wilkerson JL, Alberti LB, Thakur GA, Makriyannis A, Milligan ED. Peripherally administered cannabinoid receptor 2 (CB2R) agonists lose anti-allodynic effects in TRPV1 knockout mice, while intrathecal administration leads to anti-allodynia and reduced GFAP, CCL2 and TRPV1 expression in the dorsal spinal cord and DRG. Brain Res. 2022;1774:147721.
Newton AC. Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol. 2018;53:208–30.
Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circul Res. 2010;106:1319–31.
Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–90.
Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–27.
Pabbidi RM, Yu S-Q, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:1–17.
Ramírez-Coronel AA, Abdu WJ, Alshahrani SH, Treve M, Jalil AT, Alkhayyat AS, Singer N. Childhood obesity risk increases with increased screen time: a systematic review and dose–response meta-analysis. J Health Popul Nutr. 2023;42(1):5.
Lam D, Momeni Z, Theaker M, Jagadeeshan S, Yamamoto Y, Ianowski JP, et al. RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS ONE. 2018;13:e0193312.
Johnson K, Doucette A, Edwards A, Watts VJ, Klein AH. Reduced activity of Adenylyl Cyclase 1 Attenuates Morphine Induced Hyperalgesia and Inflammatory Pain in Mice. 2020.
Herrmann S, Rajab H, Christ I, Schirdewahn C, Höfler D, Fischer MJ, et al. Protein kinase a regulates inflammatory pain sensitization by modulating HCN2 channel activity in nociceptive sensory neurons. Pain. 2017;158:2012–24.
Kareem AA, Sabhan AH. The significant relationship between a prostatitis and a high Level of glycated hemoglobin in non-Insulin dependent Diabetes Mellitus in Al-Diwaniah Province. J. Biomed. Biochem. 2022:1(2):25–28. https://doi.org/10.57238/jbb.2022.5455.1007
Xu X, Ren Z, Tang Y, Zilundu PL, Zhou Y, Li W et al. Dexmedetomidine attenuates hyperalgesia induced by brachial plexus root avulsion by restoring the GLT-1 function via PKA signaling. 2022.
Bhave G, Zhu W, Wang H, Brasier D, Oxford GS, Gereau IVRW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–31.
Gunn RM, Hailes HC. Insights into the PI3-K-PKB-mTOR signalling pathway from small molecules. J Chem Biol. 2008;1:49–62.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.
Guo J-R, Wang H, ** X-J, Jia D-L, Zhou X, Tao Q. Effect and mechanism of inhibition of PI3K/Akt/mTOR signal pathway on chronic neuropathic pain and spinal microglia in a rat model of chronic constriction injury. Oncotarget. 2017;8:52923.
Chen H, Hu Y, **e K, Chen Y, Wang H, Bian Y, et al. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int J Mol Med. 2018;42:2009–19.
Liu K, Yang Y, Zhou F, **ao Y, Shi L. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy and relieves hyperalgesia in diabetic rats. NeuroReport. 2020;31:644–9.
Bayer KU, Schulman H. CaM kinase: still inspiring at 40. Neuron. 2019;103:380–94.
Qian Y, **a T, Cui Y, Chu S, Ma Z, Gu X. The role of CaMKII in neuropathic pain and fear memory in chronic constriction injury in rats. Int J Neurosci. 2019;129:148–56.
Yu H, Pan B, Weyer A, Wu H-E, Meng J, Fischer G, et al. CaMKII controls whether touch is painful. J Neurosci. 2015;35:14086–102.
Morris EP, Török K. Oligomeric structure of α-calmodulin-dependent protein kinase II. J Mol Biol. 2001;308:1–8.
Boric M, Kadic AJ, Puljak L. The expression of calcium/calmodulin-dependent protein kinase II in the dorsal horns of rats with type 1 and type 2 diabetes. Neurosci Lett. 2014;579:151–6.
Ferhatovic L, Banozic A, Kostic S, Kurir TT, Novak A, Vrdoljak L, et al. Expression of calcium/calmodulin-dependent protein kinase II and pain-related behavior in rat models of type 1 and type 2 diabetes. Anesth Analgesia. 2013;116:712–21.
Jerić M, Vuica A, Borić M, Puljak L, Kadić AJ, Grković I, et al. Diabetes mellitus affects activity of calcium/calmodulin-dependent protein kinase II alpha in rat trigeminal ganglia. J Chem Neuroanat. 2015;64:12–9.
Bian H, Yu L-C. Intra-nucleus accumbens administration of the calcium/calmodulin-dependent protein kinase II inhibitor AIP induced antinociception in rats with mononeuropathy. Neurosci Lett. 2015;599:129–32.
He X-f, Kang Y-r, Fei X-y, Chen L-h, Li X, Ma Y-q et al. Inhibition of phosphorylated calcium/calmodulin-dependent protein kinase IIα relieves streptozotocin-induced diabetic neuropathic pain through regulation of P2X3 receptor in dorsal root ganglia.Purinergic Signalling2022:1–13.
Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, et al. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003;90:2098–105.
Matsumura S, Kunori S, Mabuchi T, Katano T, Nakazawa T, Abe T, et al. Impairment of CaMKII activation and attenuation of neuropathic pain in mice lacking NR2B phosphorylated at Tyr1472. Eur J Neurosci. 2010;32:798–810.
Omear HA. Novel SNPs of TNF-a and IL-6 that Regulate Serum Level in Obese Patients. J. Biomed. Biochem. 2023:2(1):7–20. https://doi.org/10.57238/jbb.2023.6398.1025
Lee J, Saloman JL, Weiland G, Auh Q-S, Chung M-K, Ro JY. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain. 2012;153:1514–24.
Guo S-H, Lin J-P, Huang L-E, Yang Y, Chen C-Q, Li N-N, et al. Silencing of spinal Trpv1 attenuates neuropathic pain in rats by inhibiting CAMKII expression and ERK2 phosphorylation. Sci Rep. 2019;9:1–9.
Zhao X, Shen L, Xu L, Wang Z, Ma C, Huang Y. Inhibition of CaMKIV relieves streptozotocin-induced diabetic neuropathic pain through regulation of HMGB1. BMC Anesthesiol. 2015;16:1–8.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Mustafa Gheni Taher and Mazin Razooqi Mohammed gave the concept. Muthanna Abdulkhader Salh Al-Mahdawi and Noor Kareem Assi Halaf wrote the manuscript. Abduladheem Turki Jalil prepared the figure. Tahani Alsandook scientifically revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to publish
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taher, M.G., Mohammed, M.R., Al-Mahdawi, M.A.S. et al. The role of protein kinases in diabetic neuropathic pain: an update review. J Diabetes Metab Disord 22, 147–154 (2023). https://doi.org/10.1007/s40200-023-01217-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01217-1