Abstract
Background
The global prevalence of type 2 diabetes mellitus (T2DM) raises the rates of its complications, such as diabetic nephropathy and cardiovascular diseases. To conquer the complications, new strategies to reverse the deterioration of T2DM are urgently needed. In this project, we aimed to examine the hypoglycemic effect of primaquine and explore its specific target.
Methods
In vitro T2DM insulin resistance model was built in HepG2 cells to screen the potential anti-diabetic chemicals. On the other hand, the potential protein targets were explored by molecular docking. Accordingly, we chose C57BL/6 N mice to establish T2DM model to verify the effect of the chemicals on anti-hyperglycemia and diabetic complications.
Results
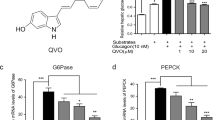
By targeting the Keratin 7 (K7) to activate EGFR/Akt glucose metabolism signaling pathway, primaquine poses a potent hypoglycemic effect. The level of acetyl-CoA is enhanced markedly, supporting that primaquine upregulates the aerobic glycolysis. Moreover, primaquine ameliorates kidney function by reducing the secretion of urinary proteins and creatinine, especially for the urea nitrogen which is significantly decreased compared to no-treatment T2DM mice. Notably, primaquine restores the level of plasma low-density lipoprotein cholesterol (LDL-C) nearly to normal, minimizing the incidence of cardiovascular diseases.
Conclusions
We find that primaquine may reverse the dysregulated metabolism to prevent diabetic complications by stimulating EGFR/Akt signaling axis, shedding new light on the therapy of T2DM.
Graphical abstract

Insulin resistance is characterized by reduced p-Akt and glucose metabolism, dominated by anaerobic glycolysis. Primaquine activates the complex made of K7 and EGFR, further stimulating Akt phosphorylation. Then, p-Akt promotes the aerobic glucose metabolism and upregulates Ac-CoA to mobilize TCA cycle, improving insulin sensitivity.




Similar content being viewed by others
Abbreviations
- Ac-CoA:
-
Acetyl-CoA
- DCVD:
-
Diabetic cardiovascular disease
- DN:
-
Diabetic nephropathy
- EGFR:
-
Epidermal growth factor
- FFA:
-
Free fatty acids
- HbA1c:
-
Glycated hemoglobin A1c
- HFD:
-
High fat diet
- HOMA-IR:
-
Homeostasis model of assessment-insulin resistance
- IPGTT:
-
Intraperitoneal glucose tolerance test
- IPITT:
-
Intraperitoneal insulin tolerance test
- K7:
-
Keratin 7
- LDH:
-
Lactic dehydrogenase
- LDL-C:
-
Low-density lipoprotein cholesterol
- MDA:
-
Malondialdehyde
- MG:
-
Methylglyoxal
- PB:
-
Primaquine phosphate
- SD:
-
Standard Diet
- STZ:
-
Streptozotocin
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TCA:
-
Tricarboxylic acid cycle
- T-SOD:
-
Total superoxide dismutase
- VLDL:
-
Very low-density lipoprotein
References
Jenkins AJ, et al. Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015;12(1–2):159–95.
Ogurtsova K, et al. IDF diabetes atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118.
Kalluri SR, et al. Do SGLT2 inhibitors improve cardio-renal outcomes in patients with type II diabetes mellitus: a systematic review. Cureus. 2021;13(9):e17668.
Mertowska P, et al. A link between chronic kidney disease and gut microbiota in immunological and nutritional aspects. Nutrients. 2021;13(10):1–23.
Sharma K. Mitochondrial dysfunction in the diabetic kidney. In: Santulli G, editor. Mitochondrial dynamics in cardiovascular medicine. Cham: Springer International Publishing; 2017. p. 553–62.
Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease: with emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Supplement_2):64–78.
Antar SA, et al. Telmisartan attenuates diabetic nephropathy by mitigating oxidative stress and inflammation, and upregulating Nrf2/HO-1 signaling in diabetic rats. Life Sci. 2021;291:120260.
Thambiah SC, Lai LC. Diabetic dyslipidaemia. Pract Lab Med. 2021;26:e00248.
Hirano T. Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb. 2018;25(9):771–82.
Kourtidou C, et al. Novel cardiovascular risk factors in patients with diabetic kidney disease. Int J Mol Sci. 2021;22(20):1–11.
Kalra S, et al. Malaria and diabetes. J Pak Med Assoc. 2017;67(5):810–3.
Carrillo-Larco RM, Altez-Fernandez C, Ugarte-Gil C. Is diabetes associated with malaria and malaria severity? A systematic review of observational studies. Wellcome Open Res. 2019;4:136.
Vera IM, et al. Targeting liver stage malaria with metformin. JCI Insight. 2019;4(24):1–10.
Sheikhbahaie F, et al. The effect of hydroxychloroquine on glucose control and insulin resistance in the prediabetes condition. Adv Biomed Res. 2016;5:145.
Heard CM, Monk BV, Modley AJ. Binding of primaquine to epidermal membranes and keratin. Int J Pharm. 2003;257(1–2):237–44.
Alam CM, et al. Keratin 7 is a constituent of the keratin network in mouse pancreatic islets and is upregulated in experimental diabetes. Int J Mol Sci. 2021;22(15):1–16.
Barak V, et al. Clinical utility of cytokeratins as tumor markers. Clin Biochem. 2004;37(7):529–40.
Toivola DM, et al. Keratins in health and disease. Curr Opin Cell Biol. 2015;32:73–81.
Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013;25(5):600–12.
Kim JH, et al. Enhanced glycolysis supports cell survival in EGFR-mutant lung adenocarcinoma by inhibiting autophagy-mediated EGFR degradation. Cancer Res. 2018;78(16):4482–96.
Blagoev B, et al. A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat Biotechnol. 2003;21(3):315–8.
Ru P, et al. Tumor metabolism of malignant gliomas. Cancers (Basel). 2013;5(4):1469–84.
Sefried S, et al. Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression. Open Biol. 2018;8(10):1–11.
Ding X, et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed Pharmacother. 2019;110:85–94.
Kim HK, et al. TMBIM6/BI-1 contributes to cancer progression through assembly with mTORC2 and AKT activation. Nat Commun. 2020;11(1):4012.
Srinivasan K, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
Dias IH, Griffiths HR. Oxidative stress in diabetes - circulating advanced glycation end products, lipid oxidation and vascular disease. Ann Clin Biochem. 2014;51(Pt 2):125–7.
Cheng KK, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9(5):417–27.
Coloff JL, et al. Akt-dependent glucose metabolism promotes Mcl-1 synthesis to maintain cell survival and resistance to Bcl-2 inhibition. Cancer Res. 2011;71(15):5204–13.
Ndisang JF, Rastogi S, Vannacci A. Insulin resistance, type 1 and type 2 diabetes, and related complications 2015. J Diabetes Res. 2015;2015:234135.
Martin-Timon I, et al. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444–70.
Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–62.
Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med 2002;251(2):87–101.
Cameron NE, et al. Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia. 1994;37(5):449–59.
Recht J, Ashley EA, White NJ. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis. 2018;12(4):e0006230.
Acknowledgments
This research was bountifully supported by “Double First-Class” university project funding from Lanzhou University (561119201) and Fundamental Research Funds for the Central Universities (grant number lzujbky-2021-kb05). We express our sincere gratitude to the Center for Experimental Animals of Lanzhou University for their support.
Author information
Authors and Affiliations
Contributions
T.W. and C.L. contributed to most of the experiments and drafted the manuscript. J.Z. and L.H. developed and established the molecular experiment. S.Q. designed the molecular docking model. J.L. mainly responsible for in vitro technology guidance. X.L. and Z.H. helped with the most of in vivo mouse studies. W.Z. provided statistical advice. X.C. conceived the project, designed the studies, solved the experimental problems, helped analyze the results, and revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
X.C., T.W., C.L., J.Z., and X.L. have applied for a patent, and we plan to benefit from the commercialization of the technology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 455 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, T., Li, C., Zhou, J. et al. Primaquine activates Keratin 7 to treat diabetes and its complications. J Diabetes Metab Disord 21, 1731–1741 (2022). https://doi.org/10.1007/s40200-022-01135-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01135-8