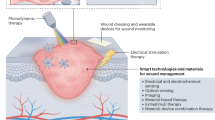
Abstract
Ever-increasing demands on improving efficiencies of wound healing procedures are a strong driving force for the development of replacement approaches. This review focuses on wound healing management from the point of formation to the point of healing procedures. The most important usual healing modality with key characteristic is explained and their limitations are discussed. Novel interesting approaches are presented with a concentration of the unique features and action mechanisms. Special attention is paid to gas plasma and nanotechnology impact on wound healing management from fundamental processes to beneficial outcomes. Challenges and opportunities for the future trend that combined common protocols and emerging technologies are discussed.






Similar content being viewed by others
References
Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition. 2010;26(9):862–6. https://doi.org/10.1016/j.nut.2010.05.008.
Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63(5):866–81. https://doi.org/10.1016/j.jaad.2009.10.048.
Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, Carrillo-Poveda JM, Cuervo-Serrato B, Peláez-Gorrea P, Sopena-Juncosa JJ. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018;9(1):10. https://doi.org/10.3390/jfb9010010.
Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, et al. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:111. https://doi.org/10.1186/s13287-019-1212-2.
Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, Lajevardi SS, Li Z, Maitz PK. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;1(123):3–17. https://doi.org/10.1016/j.addr.2017.09.018.
Powers JG, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatol Ther. 2013;26(3):197–206. https://doi.org/10.1111/dth.12055.
Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014;22(2):174–86. https://doi.org/10.1111/wrr.12125.
Okur ME, Karantas ID, Şenyiğit Z, Okur NÜ, Siafaka PI. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J Pharm Sci. 2020. https://doi.org/10.1016/j.ajps.2019.11.008.
Kalashnikova I, Das S, Seal S. Nanomaterials for wound healing: scope and advancement. Nanomedicine. 2015;10(16):2593–612. https://doi.org/10.2217/nnm.15.82.
Sharifi S. Mohammad Javad Hajipour, Lisa Gould, and Morteza Mahmoudi. Mol Pharm. 2021;18(2):550–75. https://doi.org/10.1021/acs.molpharmaceut.0c00346.
Hamdan S, Pastar I, Drakulich S, Dikici E, Tomic-Canic M, Deo S, Daunert S. Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci. 2017;3(3):163–75. https://doi.org/10.1021/acscentsci.6b00371.
Alberti TS, Coelho D, Voytena A, Pitz H, de Pra M, Mazzarino L, Kuhnen S, M Ribeiro-do-Valle R, Maraschin M, Veleirinho B. Nanotechnology: a promising tool towards wound healing. Current Pharmaceut Des. 2017;23(24):3515–28. https://doi.org/10.2174/1381612823666170503152550
Rajendran NK, Kumar SS, Houreld NN, Abrahamse H. A review on nanoparticle based treatment for wound healing. J Drug Deliv Sci Technol. 2018;1(44):421–30. https://doi.org/10.1016/j.jddst.2018.01.009.
Bekeschus S, Schmidt A, Weltmann KD, von Woedtke T. The plasma jet kINPen–A powerful tool for wound healing. Clin Plasma Med. 2016;4(1):19–28. https://doi.org/10.1016/j.cpme.2016.01.001.
Schmidt A, Liebelt G, Nießner F, von Woedtke T, Bekeschus S. Gas plasma-spurred wound healing is accompanied by regulation of focal adhesion, matrix remodeling, and tissue oxygenation. Redox Biol. 2021;1(38):101809. https://doi.org/10.1016/j.redox.2020.101809.
Arany PR. Craniofacial wound healing with photobiomodulation therapy: new insights and current challenges. J Dent Res. 2016;95(9):977–84. https://doi.org/10.1177/0022034516648939.
Mohseni S, Aalaa M, Atlasi R, et al. The effectiveness of negative pressure wound therapy as a novel management of diabetic foot ulcers: an overview of systematic reviews. J Diabetes Metab Disord. 2019;18:625–41. https://doi.org/10.1007/s40200-019-00447-6.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. https://doi.org/10.1056/NEJM199909023411006.
Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370–8. https://doi.org/10.1111/bjd.13954.
Werdin F, Tenenhaus M, Rennekampff HO. Chronic wound care. Lancet. 2008;372(9653):1860–2. https://doi.org/10.1016/S0140-6736(08)61793-6.
Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: an updated review. J Dermatol Treat. 2020;31(6):639–48. https://doi.org/10.1080/09546634.2018.1530443.
Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ. 2002;324(7330):160–3. https://doi.org/10.1136/bmj.324.7330.160.
Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005;26(4):306–19. https://doi.org/10.1097/01.BCR.0000169887.04973.3A.
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560–82. https://doi.org/10.1089/wound.2015.0635.
Guo SA, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. https://doi.org/10.1177/0022034509359125.
Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72(3):206–17. https://doi.org/10.1016/j.jdermsci.2013.07.008.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–42. https://doi.org/10.1177/147323000903700531.
Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Investig. 2007;117(5):1219–22. https://doi.org/10.1172/JCI32169.
George Broughton II, Janis JE, Attinger CE. Wound healing: an overview. Plastic Reconstruct Surg. 2006;117(7S):1e-S. https://doi.org/10.1097/01.prs.0000222562.60260.f9.
Gethin G. Understanding the inflammatory process in wound healing. British journal of community nursing. 2012;17(Sup3):S17–22. https://doi.org/10.12968/bjcn.2012.17.Sup3.S17
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x.
Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218(11):1376–84. https://doi.org/10.1016/j.imbio.2013.06.005.
Fujiwara N, Kobayashi K. Macrophages in inflammation. Current Drug Targets Inflamm Allergy. 2005;4(3):281–6. https://doi.org/10.2174/1568010054022024.
Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–85. https://doi.org/10.1007/s00018-016-2268-0.
Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. In Seminars in cell & developmental biology. vol. 61. Academic Press; 2017. p. 3–11. https://doi.org/10.1016/j.semcdb.2016.08.006
Serra MB, Barroso WA, Silva NN, Silva SD, Borges AC, Abreu IC, Borges MO. From inflammation to current and alternative therapies involved in wound healing. Int J Inflamm. 2017. https://doi.org/10.1155/2017/3406215
Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep. 2005;57:108.
Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol WJG. 2011;17(17):2161. https://doi.org/10.3748/wjg.v17.i17.2161.
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, Guan H, Liu J, Shi J, Su L, Hu D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol. 2017;48(2):121–32. https://doi.org/10.1007/s10735-017-9711-x.
Liu CY, Kao WW. Corneal epithelial wound healing. Prog Mol Biol Transl Sci. 2015;1(134):61–71. https://doi.org/10.1016/bs.pmbts.2015.05.002.
Häkkinen L, Larjava H, Koivisto L. Granulation tissue formation and remodeling. Endod Top. 2011;24(1):94–129. https://doi.org/10.1111/etp.12008.
Berlanga-Acosta J, Schultz GS, López-Mola E, Guillen-Nieto G, García-Siverio M, Herrera-Martínez L. Glucose toxic effects on granulation tissue productive cells: the diabetics’ impaired healing. BioMed Res Int. 2013. https://doi.org/10.1155/2013/256043
Douglas HE. TGF-β in wound healing: a review. J Wound Care. 2010;19(9):403–6. https://doi.org/10.12968/jowc.2010.19.9.78235.
Teller P, White TK. The physiology of wound healing: injury through maturation. Perioper Nursing Clin. 2011;6(2):159–70. https://doi.org/10.1016/j.cpen.2011.04.001.
Rognoni E, Pisco AO, Hiratsuka T, Sipilä KH, Belmonte JM, Mobasseri SA, Philippeos C, Dilão R, Watt FM. Fibroblast state switching orchestrates dermal maturation and wound healing. Molecular Syst Biol. 2018;14(8):e8174. https://doi.org/10.15252/msb.20178174.
Potter M, Banwell P, Baldwin C, Clayton E, Irvine L, Linge C, et al. In vitro optimisation of topical negative pressure regimens for angiogenesis into synthetic dermal replacements. Burns. 2008;34(2):164–74. https://doi.org/10.1016/j.burns.2007.06.020.
Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301–31. https://doi.org/10.1007/978-3-319-66990-8_12.
Uhl E, Sirsjö A, Haapaniemi T, Nilsson G, Nylander G. Hyperbaric oxygen improves wound healing in normal and ischemic skin tissue. Plast Reconstr Surg. 1994;93(4):835–41.
Duzgun AP, Satır HZ, Ozozan O, Saylam B, Kulah B, Coskun F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot Ankle Surg. 2008;47(6):515–9. https://doi.org/10.1053/j.jfas.2008.08.002.
Weiser JR, Saltzman WM. Controlled release for local delivery of drugs: barriers and models. J Controlled Release. 2014;190:664–73. https://doi.org/10.1016/j.jconrel.2014.04.048.
Sussman C, Bates-Jensen BM. Wound care: a collaborative practice manual. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
Gunavathi P. Development of combined wound dressings made of nylon-6/PCL nanomembrane. In: Shodhganga, editor. Wound dressings. 2015. p. 119–40.
Rueda Lopez J, Arboix Perejamo M, Munoz Bueno AM, Rosell Moreno C, Blanco Blanco J, Ballester Torralba J, et al. Combined polyurethane foam and hydrogel dressing. Outcome in lesions of diverse etiology. Revista de Enfermeria. 2004;27(11):51–6.
Maver T, Gradišnik L, Kureˇciˇc M, Hribernik S, Smrke DM, Maver U, et al. Layering of different materials to achieve optimal conditions for treatment of painful wounds. Int J Pharm. 2017;529(1–2):576–88. https://doi.org/10.1016/j.ijpharm.2017.07.043.
Jaklic D, Lapanje A, Zupancic K, Smrke D, Gunde-Cimerman N. Selective antimicrobial activity of maggots against pathogenic bacteria. J Med Microbiol. 2008;57(Pt 5):617–25. https://doi.org/10.1099/jmm.0.47515-0.
Shi L, Carson D. Collagenase Santyl ointment: a selective agent for wound debridement. J Wound Ostomy Continence Nurs. 2009;36(6S):S12–6. https://doi.org/10.1097/WON.0b013e3181bfdd1a.
Sherman RA. Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol. 2009;3(2):336–44. https://doi.org/10.1177/193229680900300215.
Weiss EA, Oldham G, Lin M, Foster T, Quinn JV. Water is a safe and effective alternative to sterile normal saline for wound irrigation prior to suturing: a prospective, double-blind, randomised, controlled clinical trial. BMJ Open. 2013;3(1). http://dx.doi.org/https://doi.org/10.1136/bmjopen-2012-001504
Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP, Lund PA, Oppenheim BA, Webber MA. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLoS ONE. 2015;10(9):e0136190. https://doi.org/10.1371/journal.pone.0136190.
Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent patents on anti-infective drug discovery. 2010;5(2):124–51. https://doi.org/10.2174/157489110791233522
Verbanic S, Shen Y, Lee J, Deacon JM, Chen IA. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiom. 2020;6(1):1–11. https://doi.org/10.1038/s41522-020-0130-5.
Madhok BM, Vowden K, Vowden P. New techniques for wound debridement. Int Wound J. 2013;10(3):247–51. https://doi.org/10.1111/iwj.12045.
Falabella AF. Debridement and wound bed preparation. Dermatol Ther. 2006;19(6):317–25. https://doi.org/10.1111/j.1529-8019.2006.00090.x.
Simões D, Miguel SP, Ribeiro MP, Coutinho P, Mendonça AG, Correia IJ. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;1(127):130–41. https://doi.org/10.1016/j.ejpb.2018.02.022.
Dhivya S, Padma VV, Santhini E. Wound dressings—a review. BioMedicine. 2015;5(4). https://doi.org/10.7603/s40681-015-0022-9
Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21(2):77–95. https://doi.org/10.1002/pat.1625.
Thu HE, Zulfakar MH, Ng SF. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int J Pharm. 2012;434(1–2):375–83. https://doi.org/10.1016/j.ijpharm.2012.05.044.
Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: a mini review. Carbohyd Polym. 2020;15(236): 116025. https://doi.org/10.1016/j.carbpol.2020.116025.
Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26(32):6335–42. https://doi.org/10.1016/j.biomaterials.2005.04.012.
** SG, Yousaf AM, Kim KS, Kim DW, Kim DS, Kim JK, Yong CS, Youn YS, Kim JO, Choi HG. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int J Pharm. 2016;501(1–2):160–6. https://doi.org/10.1016/j.ijpharm.2016.01.044.
Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine—challenge and perspectives. Angew Chem Int Ed. 2009;48(5):872–97. https://doi.org/10.1002/anie.200802585.
Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle-based dressing: the future of wound treatment? Trends Biotechnol. 2017;35(8):770–84. https://doi.org/10.1016/j.tibtech.2017.05.005.
Mahmoudi M, Zhao M, Matsuura Y, Laurent S, Yang PC, Bernstein D, Ruiz-Lozano P, Serpooshan V. Infection-resistant MRI-visible scaffolds for tissue engineering applications. BioImpacts. 2016;6(2):111. https://doi.org/10.15171/bi.2016.16.
Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. https://doi.org/10.1016/j.tibtech.2012.06.004.
Liu Y, Shi L, Su L, van der Mei HC, Jutte PC, Ren Y, Busscher HJ. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev. 2019;48(2):428–46. https://doi.org/10.1039/C7CS00807D.
Greulich C, Braun D, Peetsch A, Diendorf J, Siebers B, Epple M, Köller M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012;2(17):6981–7. https://doi.org/10.1039/C2RA20684F.
Khampieng T, Brikshavana P, Supaphol P. Silver nanoparticle embedded poly (vinyl pyrrolidone) hydrogel dressing: gamma-ray synthesis and biological evaluation. J Biomater Sci Polym Ed. 2014;25(8):826–42. https://doi.org/10.1080/09205063.2014.910154.
Shankar S, Jaiswal L, Aparna RS, Prasad RG, Kumar GP, Manohara CM. Wound healing potential of green synthesized silver nanoparticles prepared from Lansium domesticum 90 wound healing—new insights into ancient challenges fruit peel extract. Mater Exp. 2015;5(2):159–64. https://doi.org/10.1166/mex.2015.1225.
Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–48. https://doi.org/10.1016/j.burns.2006.06.010.
Oliveira RN, Rouzé R, Quilty B, Alves GG, Soares GD, Thiré RM, McGuinness GB. Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings. Interf Focus. 2014;4(1):20130049. https://doi.org/10.1098/rsfs.2013.0049.
Yan X, Fang WW, Xue J, Sun TC, Dong L, Zha Z, Qian H, Song YH, Zhang M, Gong X, Lu Y. Thermoresponsive in situ forming hydrogel with sol-gel irreversibility for effective methicillin-resistant Staphylococcus aureus infected wound healing. ACS Nano. 2019;13(9):10074–84. https://doi.org/10.1021/acsnano.9b02845.
Miao J, Pangule RC, Paskaleva EE, Hwang EE, Kane RS, Linhardt RJ, Dordick JS. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials. 2011;32(36):9557–67. https://doi.org/10.1016/j.biomaterials.2011.08.080.
Pivec T, Hribernik S, Ribitsch V, Stana-Kleinschek K, Fzs PL, et al. Antimicrobial cellulose material and process of its production: European Patent Application No. EP13151727.8, 17 (referenceP003373EP), SubmissionNumber1966536:Eu päischesPatentamt; 2013
Pivec T, Hribernik S, Kolar M, Kleinschek KS. Environmentally friendly procedure for in-situ coating of regenerated cellulose fibres with silver nanoparticles. Carbohyd Polym. 2017;1(163):92–100.
Mao C, **ang Y, Liu X, Cui Z, Yang X, Yeung KW, Pan H, Wang X, Chu PK, Wu S. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@ AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010–21. https://doi.org/10.1021/acsnano.7b03513.
Yuwen L, Sun Y, Tan G, **u W, Zhang Y, Weng L, Teng Z, Wang L. MoS 2@ polydopamine-Ag nanosheets with enhanced antibacterial activity for effective treatment of Staphylococcus aureus biofilms and wound infection. Nanoscale. 2018;10(35):16711–20. https://doi.org/10.1039/C8NR04111C.
Qiao Y, He J, Chen W, Yu Y, Li W, Du Z, **e T, Ye Y, Hua SY, Zhong D, Yao K. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14(3):3299–315. https://doi.org/10.1021/acsnano.9b08930.
Li M, Liu X, Tan L, Cui Z, Yang X, Li Z, Zheng Y, Yeung KW, Chu PK, Wu S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater Sci. 2018;6(8):2110–21. https://doi.org/10.1039/C8BM00499D.
Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, Chamilos G, Feldmeyer L, Marinari B, Chon S, Vence L. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5(1):1–6. https://doi.org/10.1038/ncomms6621.
Fumakia M, Ho EA. Nanoparticles encapsulated with LL37 and serpin A1 promotes wound healing and synergistically enhances antibacterial activity. Mol Pharm. 2016;13(7):2318–31. https://doi.org/10.1021/acs.molpharmaceut.6b00099.
Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs. 2012;26(3):163–75. https://doi.org/10.2165/11631850-000000000-0000.
Hardwicke J, Ferguson EL, Moseley R, Stephens P, Thomas DW, Duncan R. Dextrin–rhEGF conjugates as bioresponsive nanomedicines for wound repair. J Control Release. 2008;130(3):275–83. https://doi.org/10.1016/j.jconrel.2008.07.023.
Fabiilli ML, Wilson CG, Padilla F, Martín-Saavedra FM, Fowlkes JB, Franceschi RT. Acoustic droplet–hydrogel composites for spatial and temporal control of growth factor delivery and scaffold stiffness. Acta Biomater. 2013;9(7):7399–409. https://doi.org/10.1016/j.actbio.2013.03.027.
Johnson NR, Wang Y. Controlled delivery of heparin-binding EGF-like growth factor yields fast and comprehensive wound healing. J Control Release. 2013;166(2):124–9. https://doi.org/10.1016/j.jconrel.2012.11.004.
Lin YK, Chen KH, Ou KL, Liu M. Effects of different extracellular matrices and growth factor immobilization on biodegradability and biocompatibility of macroporous bacterial cellulose. J Bioact Compat Polym. 2011;26(5):508–18. https://doi.org/10.1177/0883911511415390.
Ribeiro MP, Morgado PI, Miguel SP, Coutinho P, Correia IJ. Dextran based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C. 2013;33(5):2958–66. https://doi.org/10.1016/j.msec.2013.03.025.
Zhang M, Rehman J, Malik AB. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21(3):224. https://doi.org/10.1097/MOH.0000000000000041.
Hassan W, Dong Y, Wang W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res Ther. 2013;4(2):32. https://doi.org/10.1186/scrt182.
Peng LH, Wei W, Qi XT, Shan YH, Zhang FJ, Chen X, Zhu QY, Yu L, Liang WQ, Gao JQ. Epidermal stem cells manipulated by pDNA-VEGF165/CYD-PEI nanoparticles loaded gelatin/β-TCP matrix as a therapeutic agent and gene delivery vehicle for wound healing. Mol Pharm. 2013;10(8):3090–102. https://doi.org/10.1021/mp400162k.
Im GB, Kim YH, Kim YJ, Kim SW, Jung E, Jeong GJ, Wang K, Kim J, Kim DI, Kim TH, Yi GR. Enhancing the wound healing effect of conditioned medium collected from mesenchymal stem cells with high passage number using bioreducible nanoparticles. Int J Mol Sci. 2019;20(19):4835. https://doi.org/10.3390/ijms20194835.
Rasouli M, Fallah N, Ostrikov K. Lung cancer oncotherapy through novel modalities: Gas plasma and nanoparticle technologies. IntechOpen. 2020. https://doi.org/10.5772/intechopen.95494.
Rasouli M, Fallah N, Ostrikov K. Lung cancer oncotherapy through novel modalities: gas plasma and nanoparticle technologies [online first]. IntechOpen. 2021. https://doi.org/10.5772/intechopen.95494.
Rasouli M, Mehdian H, Hajisharifi K, Amini E, Ostrikov K, Robert E. Plasma processes polym. 2021;e2100074. https://doi.org/10.1002/ppap.202100074.
Schmidt A, Bekeschus S. Redox for repair: cold physical plasmas and nrf2 signaling promoting wound healing. Antioxidants. 2018;7(10):146. https://doi.org/10.3390/antiox7100146.
Lu X, Naidis GV, Laroussi M, Reuter S, Graves DB, Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Phys Rep. 2016;4(630):1–84. https://doi.org/10.1016/j.physrep.2016.03.003.
Stratmann B, Costea TC, Nolte C, Hiller J, Schmidt J, Reindel J, Masur K, Motz W, Timm J, Kerner W, Tschoepe D. Effect of cold atmospheric plasma therapy vs standard therapy placebo on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw Open. 2020;3(7):e2010411. https://doi.org/10.1001/jamanetworkopen.2020.10411.
Reuter S, Von Woedtke T, Weltmann KD. The kINPen—a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D Appl Phys. 2018;51(23): 233001. https://doi.org/10.1088/1361-6463/aab3ad.
Von Woedtke T, Reuter S, Masur K, Weltmann KD. Plasmas for medicine. Phys Rep. 2013;530(4):291–320. https://doi.org/10.1016/j.physrep.2013.05.005.
Lu X, Keidar M, Laroussi M, Choi E, Szili EJ, Ostrikov K. Transcutaneous plasma stress: from soft-matter models to living tissues. Mater Sci Eng R Rep. 2019;1(138):36–59. https://doi.org/10.1016/j.mser.2019.04.002.
Lloyd G, Friedman G, Jafri S, Schultz G, Fridman A, Harding K. Gas plasma: medical uses and developments in wound care. Plasma Process Polym. 2010;7(3–4):194–211. https://doi.org/10.1002/ppap.200900097.
Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann Biomed Eng. 2010;38(3):748–57. https://doi.org/10.1007/s10439-009-9868-x.
Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, Vasilets V, Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma Process. 2007;27(2):163–76. https://doi.org/10.1007/s11090-007-9048-4.
Braný D, Dvorská D, Halašová E, Škovierová H. Cold atmospheric plasma: a powerful tool for modern medicine. Int J Mol Sci. 2020;21(8):2932. https://doi.org/10.3390/ijms21082932.
Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, Monetti R, Steffes B, Bunk W, Li Y, Klaempfl T. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. Br J Dermatol. 2012;167(2):404–10. https://doi.org/10.1111/j.1365-2133.2012.10923.x.
Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B, Bunk W. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010;163(1):78–82. https://doi.org/10.1111/j.1365-2133.2010.09744.x.
Heinlin J, Isbary G, Stolz W, Zeman F, Landthaler M, Morfill G, Shimizu T, Zimmermann JL, Karrer S. A randomized two-sided placebo-controlled study on the efficacy and safety of atmospheric non-thermal argon plasma for pruritus. J Eur Acad Dermatol Venereol. 2013;27(3):324–31. https://doi.org/10.1111/j.1468-3083.2011.04395.x.
Isbary G, Stolz W, Shimizu T, et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: results of an open retrospective randomized controlled study in vivo. Clin Plasma Med. 2013;1:25–30. https://doi.org/10.1016/j.cpme.2013.06.001.
Mirpour S, Fathollah S, Mansouri P, Larijani B, Ghoranneviss M, Tehrani MM, Amini MR. Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: a randomized clinical trial. Sci Rep. 2020;10(1):1–9. https://doi.org/10.1038/s41598-020-67232-x.
Arndt S, Unger P, Berneburg M, Bosserhoff AK, Karrer S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J Dermatol Sci. 2018;89(2):181–90. https://doi.org/10.1016/j.jdermsci.2017.11.008.
Arndt S, Unger P, Wacker E, Shimizu T, Heinlin J, Li YF, Thomas HM, Morfill GE, Zimmermann JL, Bosserhoff AK, Karrer S. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE. 2013;8(11): e79325. https://doi.org/10.1371/journal.pone.0079325.
Frescaline N, Duchesne C, Favier M, Onifarasoaniaina R, Guilbert T, Uzan G, Banzet S, Rousseau A, Lataillade JJ. Physical plasma therapy accelerates wound re-epithelialisation and enhances extracellular matrix formation in cutaneous skin grafts. J Pathol. 2020;252(4):451–64. https://doi.org/10.1002/path.5546.
Schmidt A, Liebelt G, Striesow J, Freund E, von Woedtke T, Wende K, Bekeschus S. The molecular and physiological consequences of cold plasma treatment in murine skin and its barrier function. Free Radical Biol Med. 2020;1(161):32–49. https://doi.org/10.1016/j.freeradbiomed.2020.09.026.
Duchesne C, Banzet S, Lataillade JJ, Rousseau A, Frescaline N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J Pathol. 2019;249(3):368–80. https://doi.org/10.1002/path.5323.
Amini M, Sheikh Hosseini M, Fatollah S, et al. Beneficial effects of cold atmospheric plasma on inflammatory phase of diabetic foot ulcers; a randomized clinical trial. J Diabetes Metab Disord. 2020;19:895–905. https://doi.org/10.1007/s40200-020-00577-2.
Nizamoglu S, Gather MC, Humar M, Choi M, Kim S, Kim KS, et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat Commun. 2016;7:10374. https://doi.org/10.1038/ncomms10374.
Humar M, Kwok SJ, Choi M, Yetisen AK, Cho S, Yun S-H. Toward biomaterial-based implantable photonic devices. Power. 2016;1:0–11. https://doi.org/10.1515/nanoph-2016-0003.
Leal-Junior A, Guo J, Min R, Fernandes AJ, Frizera A, Marques C. Photonic smart bandage for wound healing assessment. Photon Res. 2021;9(3):272–80. https://doi.org/10.1364/PRJ.410168.
Scott-Carnell LA, Siochi EJ, Leong KW. Device and method for healing wounds. Google Patents; 2010.
Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;1(122):34–47. https://doi.org/10.1016/j.biomaterials.2017.01.011.
Li M, Chen J, Shi M, Zhang H, Ma PX, Guo B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem Eng J. 2019;1(375):121999. https://doi.org/10.1016/j.cej.2019.121999.
Tang P, Han L, Li P, Jia Z, Wang K, Zhang H, Tan H, Guo T, Lu X. Mussel-inspired electroactive and antioxidative scaffolds with incorporation of polydopamine-reduced graphene oxide for enhancing skin wound healing. ACS Appl Mater Interf. 2019;11(8):7703–14. https://doi.org/10.1021/acsami.8b18931.
Mao L, Hu S, Gao Y, Wang L, Zhao W, Fu L, Cheng H, **a L, **e S, Ye W, Shi Z. Biodegradable and electroactive regenerated bacterial cellulose/MXene (Ti3C2Tx) composite hydrogel as wound dressing for accelerating skin wound healing under electrical stimulation. Adv Healthcare Mater. 2020;9(19):2000872. https://doi.org/10.1002/adhm.202000872.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fallah, N., Rasouli, M. & Amini, M.R. The current and advanced therapeutic modalities for wound healing management. J Diabetes Metab Disord 20, 1883–1899 (2021). https://doi.org/10.1007/s40200-021-00868-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00868-2