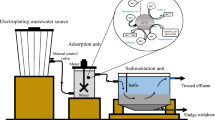
Abstract
The removal of phenol from industrial wastewater has been made more efficient by using a surface-modified nano-zero-valent iron (nZVI) catalyst produced through extended ball milling technique. This was validated through measurements such as powdered XRD and FESEM. The study utilized a central composite design (CCD) to optimize the experimental settings and create a statistical model that examined the impact of four independent process parameters, including nZVI dosage, initial phenol concentration, pH, and hydrogen peroxide concentration. The results showed that at the optimal parameter values of 0.36 g/L nZVI dosage, 1.95 mM initial phenol concentration, 1.90 mM hydrogen peroxide dose, and pH 7, a maximum removal efficiency of 91.01% was achieved within 30 min.









Similar content being viewed by others
Data Availability
The relevant source can be consulted for the information that backs up the study's conclusions.on the author's reasonable request.
References
M. Saleh, M. Yalvac, H. Arslan, N. Dizge, Investigation of basalt properties as heterogeneous catalyst for Fenton oxidation of textile wastewater. Clean-Soil, Air, Water. 50(1), 2000432 (2022). https://doi.org/10.1002/clen.202000432
B. Gunawardana, N. Singhal, P. Swedlund, Degradation of chlorinated phenols by zero valent iron and bimetals of iron: a review. Environ. Eng. Res 16(4), 187–203 (2011). https://doi.org/10.4491/eer.2011.16.4.187
A. Dutta, I. Chakraborty, D. Sarkar, S. Chakrabarti, Sunlight-assisted photo-Fenton degradation of pesticide in wastewater: ecotoxicological impact on Nostoc sp Algae. Water Air Soil Pollut. 226, 1–3 (2015). https://doi.org/10.1007/s11270-015-2661-6
W.W. Anku, M.A. Mamo, P.P. Govender, Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. Book Chapter Intech Open Publ. (2017). https://doi.org/10.5772/66927
R. Saepurahman, Hashaikeh, Insight into ball milling for size reduction and nanoparticles production of H-Y zeolite. Mater. Chem. Phys. 220, 322–330 (2018). https://doi.org/10.1016/j.matchemphys.2018.08.080
S. Li, W. Yan, W.X. Zhang, Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling. Green Chem. 10, 1618–1626 (2009). https://doi.org/10.1039/B913056J
M.I. Badawy, M.Y. Ghaly, T.A. Gad-Allah, Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 194(1–3), 166–175 (2006). https://doi.org/10.1016/j.desal.2005.09.027
R. Andreozzi, V. Caprio, A. Insola, R. Marotta, Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today. 53(1), 51–59 (1999). https://doi.org/10.1016/S0920-5861(99)00102-9
A. De, A.K. De, Role of color transformation during Fenton’s oxidation of phenolic waste water in a large scale continuous stirred tank reactor (CSTR). J. Indian Chem. Soc. May. 90(5), 649–657 (2013)
A. De, A.K. De, G.S. Panda, S. Haldar, Synthesis of iron-based nanoparticles and comparison of their catalytic activity for degradation of phenolic waste water in a small-scale batch reactor. Desalin. Water Treat. 57(52), 25170–25180 (2016). https://doi.org/10.1080/19443994.2016.1147379
A. De, A.K. De, G.S. Panda, S. Haldar, Synthesis of zero valent iron nanoparticle and its application as a dephenolization agent for coke oven plant wastewater situated in West Bengal: India. J. Environ. Prog. Sustain. Energy 36(6), 1700–1708 (2017). https://doi.org/10.1002/ep.12634
E. Neyens, J. Baeyens, A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 98(1–3), 33–50 (2003)
S.P. Sun, A.T. Lemley, p-Nitrophenol degradation by a heterogeneous Fenton-like reaction on nano-magnetite: process optimization, kinetics, and degradation pathways. J. Mol. Catal. A Chem. 349(1–2), 71–79 (2011)
G. Barzegar, S. Jorfi, V. Zarezade, M. Khatebasreh, F. Mehdipour, F. Ghanbari, 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: Reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 201, 370–379 (2018). https://doi.org/10.1016/j.chemosphere.2018.02.143
T. Nandi, A. De, S. Sarkar, S. Haldar, Optimizing the degradation of dichlorvos in aqueous solution using nZVI DTPA+ H2O2 and its detection using ac conductivity measurement with the help of response surface methodology. J. Indian Chem. Soc. 99(1), 100266 (2022). https://doi.org/10.1016/j.jics.2021.100266
K. Wang, F. Lam Wood A, ld I-RHR, SC Renc C. Quadratic RSM models of processing parameters for three-layer oriented Flakeboards. 11 (2), 173–186, (1999)
S. Boudesocque, A. Mohamadou, A. Conreux, B. Marin, L. Dupont, The recovery and selective extraction of gold and platinum by novel ionic liquids. Sep. Purif. Technol. 210, 824–834 (2019). https://doi.org/10.1016/j.jfoodeng.2005.11.024
M. Borajani, M. Baharam, M.H. Banabazi, A comparative study on the optimization of the fatty acids pretreatment parameters using central composite design with response surface methodology. J. Iran. Chem. Soc. 17, 2877–2883 (2020). https://doi.org/10.1007/s13738-020-01967-2
M. Ettinger, C. Ruchhoft, R. Lishka, Sensitive 4-amino pyrine method. J. Anal. Chem. (1951). https://doi.org/10.1021/ac60060a019
H. Kušić, N. Koprivanac, A.L. Božić, I. Selanec, Photo-assisted Fenton type processes for the degradation of phenol: a kinetic study. J. Hazard. Mater. 136(3), 632–644 (2006). https://doi.org/10.1016/j.jhazmat.2005.12.046
P. Vikeslnd, A. Heathcock, R. Rebodos, A. Rikmakus, Particle size and aggregation effects on magnetite reactivity toward carbon tetrachloride. Environ. Sci. Technol 41, 5277–5283 (2007). https://doi.org/10.1021/es062082i
Acknowledgements
We are highly thankful to the Department of Chemical Engineering, University of Calcutta and Department of BSHU, Asansol Engineering College, for necessary support and granting permission to publish this work.
Funding
Only institution funding is utilized. No special grant received.
Author information
Authors and Affiliations
Contributions
AD (Avik De) contributed to laboratory-based work and paper writing. DS contributed to-RSM including mathematical modeling for phenol kinetics. AD (Asim De) contributed to-conceptualization and editing of paper. SH contributed to-characterization of nanoparticles.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest declared by any one of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De, A., De, A., Haldar, S. et al. Kinetics Study and Optimization of Phenol Degradation from Wastewater Using Surface-Modified nano-Zero-Valent Iron (nZVI). J. Inst. Eng. India Ser. A 104, 591–601 (2023). https://doi.org/10.1007/s40030-023-00748-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40030-023-00748-3