Abstract
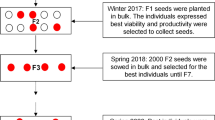
The tomato plant (Solanum lycopersicum L.) demands more water than other vegetables. However, water availability has become a limiting factor worldwide due to climate change. Thus, it is essential to explore the genetic variability of species to develop genotypes with satisfactory yields under low water availability. In this context, the objective of this study was to identify water-deficit-tolerant plants within the genetic variability of tomatoes and to select drought-tolerant genotypes from the second generation of the first backcross (F2BC1). For this, seven wild accessions, three S. lycopersicum var. cerasiforme accessions, and six commercial cultivars were tested. Moreover, intra- and interspecific crosses were performed and from the crosses S. lycopersicum × S. pennellii, two F2BC1 populations were obtained. Three experiments were conducted where the genotypes were subjected to water deficit and physiological and growth parameters. The commercial tomatoes were the most susceptible to water deficit. Among the tested cerasiform varieties, the RVC 66 accession was the least affected by the reduced water supply. The LA 716 accession (S. pennellii) had the highest tolerance to water deficit, followed by ‘LA 1401’ (S. galapagense) and ‘LA 1967’ (S. chilense). The LA 716 accession was the most promising to introgress drought tolerance-related genes in the commercial tomatoes compared to the intraspecific crosses. In addition, crossing between ‘LA 716’ and the commercial tomatoes ‘Clara’ and ‘Redenção’ allowed the development and selection of drought-tolerant F2BC1 genotypes.






Similar content being viewed by others
References
Albaladejo I, Meco V, Plasencia F, Flores FB, Bolarin MC, Egea I (2017) Unravelling the strategies used by the wild tomato species Solanum pennellii to confront salt stress: from leaf anatomical adaptations to molecular responses. Environ Exp Bot 135:1–12. https://doi.org/10.1016/j.envexpbot.2016.12.003
Ashrafi H, Kinkade M, Foolad MR (2009) A new genetic linkage map of tomato based on a Solanum lycopersicum x S. pimpinellifolium RIL population displaying locations of candidate pathogen response genes. Genome 52:935–956. https://doi.org/10.1139/G09-065
Bai Y, Lindhout P (2007) Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Ann Bot 100:1085–1094. https://doi.org/10.1093/aob/mcm150
Bedinger PA, Chetelat RT, McClure B, Moyle LC, Rose JKC, Stack SM, van der Knaap E, Baek YS, Lopez-Casado G, Covey PA, Kumar A, Li W, Nunez R, Cruz-Garcia F, Royer S (2011) Interspecific reproductive barriers in the tomato clade: opportunities to decipher mechanisms of reproductive isolation. Sex Plant Reprod 24:171–187. https://doi.org/10.1007/s00497-010-0155-7
Bergougnoux V (2014) The history of tomato: from domestication to biopharming. Biotechnol Adv 32:170–189. https://doi.org/10.1016/j.biotechadv.2013.11.003
Blanca J, Cañizares J, Cordero L, Pascual L, Diez MJ, Nuez F (2012) Variation revealed by snp genoty** and morphology provides insight into the origin of the tomato. PLoS ONE 7:e48198. https://doi.org/10.1371/journal.pone.0048198
Bolger A, Scossa F, Bolger ME, Lanz C, Maumus F, Tohge T, Quesneville H, Alseekh S, Sørensen I, Lichtenstein G, Fich EA, Conte M, Keller H, Schneeberger K, Schwacke R, Ofner I, Vrebalov J, Xu Y, Osorio S, Aflitos SA, Schijlen E, Jiménez-Goméz JM, Ryngajllo M, Kimura S, Kumar R, Koenig D, Headland LR, Maloof JN, Sinha N, van Ham RCHJ, Lankhorst RK, Mao L, Vogel A, Arsova B, Panstruga R, Fei Z, Rose JKC, Zamir D, Carrari F, Giovannoni JJ, Weigel D, Usadel B, Fernie AR (2014) The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat Genet 46:1034–1038. https://doi.org/10.1038/ng.3046
Borba MEA, Maciel GM, Fraga Júnior EF, Machado Júnior CS, Marquez GR, Silva IG, Almeida RS (2017) Gas exchanges and water use efficiency in the selection of tomato genotypes tolerant to water stress. Genet Mol Res. https://doi.org/10.4238/gmr16029685
Brog YM, Osorio S, Yichie Y, Alseekh S, Bensal E, Kochevenko A, Zamir D, Fernie AR (2019) A Solanum neorickii introgression population providing a powerful complement to the extensively characterized Solanum pennellii population. Plant J 97:391–403. https://doi.org/10.1111/tpj.14095
Carvalho CRF, Ponciano NJ, Souza PM, Souza CLM, Sousa EF (2014) Viabilidade econômica e de risco da produção de tomate no município de Cambuci/RJ, Brasil. Ciênc Rural 44:2293–2299. https://doi.org/10.1590/0103-8478cr20131570
Chetelat RT (2016) Overcoming sterility and unilateral incompatibility of Solanum lycopersicum × S. sitiens hybrids. Euphytica 207:319–330. https://doi.org/10.1007/s10681-015-1543-8
Constantinescu D, Memmah MM, Vercambre G, Génard M, Baldazzi V, Causse M, Albert E, Brunel B, Valsesia P, Bertin N (2016) Model-assisted estimation of the genetic variability in physiological parameters related to tomato fruit growth under contrasted water conditions. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01841
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58. https://doi.org/10.1038/nclimate1633
Dariva FD, Copati MGF, Pessoa HP, Alves FM, Dias FO, Picoli EAT, Cunha FF, Nick C (2020) Evaluation of anatomical and physiological traits of Solanum pennellii Cor. associated with plant yield in tomato plants under water-limited conditions. Sci Rep 10:16052. https://doi.org/10.1038/s41598-020-73004-4
Dias DM, Resende JT, Zeist AR, Gabriel A, Santos MH, Vilela NC (2019) Resistance of processing tomato genotypes to leafminer (Tuta absoluta). Hortic Bras 37:40–46. https://doi.org/10.1590/s0102-053620190106
Diouf I, Derivot L, Koussevitzky S, Carretero Y, Bitton F, Moreau L, Causse M (2020) Genetic basis of phenotypic plasticity and genotype x environment interactions in a multi-parental tomato population. J Exp Bot 71:5365–5376. https://doi.org/10.1093/jxb/eraa265
Du YL, Wang ZY, Fan JW, Turner N, He J, Wang T, Li F (2013) Exogenous abscisic acid reduces water loss and improves antioxidant defence, desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit. Funct Plant Biol 40:494–506. https://doi.org/10.1071/FP12250
Easlon HM, Richards JH (2009) Drought response in self-compatible species of tomato (Solanaceae). Am J Bot 96:605–611. https://doi.org/10.3732/ajb.0800189
Egea I, Albaladejo I, Meco V, Morales B, Sevilla A, Bolarin MC, Flores FB (2018) The drought-tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci Rep 8:2791. https://doi.org/10.1038/s41598-018-21187-2
Fischer I, Camus-Kulandaivelu L, Allal F, Stephan W (2011) Adaptation to drought in two wild tomato species: the evolution of the Asr gene family. New Phytol 190:1032–1044. https://doi.org/10.1111/j.1469-8137.2011.03648.x
Flores FB, Sanchez-Bel P, Estañ MT, Martinez-Rodriguez MM, Moyano E, Morales B, Campos JF, Garcia-Abellán JO, Egea MI, Fernández-Garcia N, Romojaro F, Bolarín MC (2010) The effectiveness of grafting to improve tomato fruit quality. Sci Hortic 125:211–217. https://doi.org/10.1016/j.scienta.2010.03.026
Galmés J, Ochogavía JM, Gago J, Roldán EJ, Cifre J, Conesa MA (2013) Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ 36:920–935. https://doi.org/10.1111/pce.12022
Gasparini K, dos Reis MJ, Peres LE, Zsögön A (2021) De novo domestication of wild species to create crops with increased resilience and nutritional value. Curr Opin Plant Biol 60:102006. https://doi.org/10.1016/j.pbi.2021.102006
Guida G, Sellami MH, Mistretta C, Oliva M, Buonomo R, De Mascellis R, Patanè C, Rouphael Y, Albrizio R, Giorio P (2017) Agronomical, physiological and fruit quality responses of two Italian long-storage tomato landraces under rain-fed and full irrigation conditions. Agric Water Manag 180:126–135. https://doi.org/10.1016/j.agwat.2016.11.004
Han W, Jia J, Hu Y, Liu J, Guo J, Shi Y, Huo H, Gong H (2020) Maintenance of root water uptake contributes to salt-tolerance of a wild tomato species under salt stress. Arch Agron Soil Sci 67:1–13. https://doi.org/10.1080/03650340.2020.1720911
Iba K (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol 53:225–245. https://doi.org/10.1146/annurev.arplant.53.100201.160729
Jenkins JA (1948) The origin of the cultivated tomato. Econ Bot 2:379–392. https://doi.org/10.1007/BF02859492
Kebede H, Martin B, Nienhuis J, King G (1994) Leaf Anatomy of two Lycopersicon species with contrasting gas exchange properties. Crop Sci 34:108–113. https://doi.org/10.2135/cropsci1994.0011183X003400010019x
Kudo M, Kidokoro S, Yoshida T, Mizoi J, Kojima M, Takebayashi Y, Sakakibara H, Fernie AR, Shinozaki K, Yamaguchi-Shinozaki K (2019) A gene-stacking approach to overcome the trade-off between drought stress tolerance and growth in Arabidopsis. Plant J 97:240–256. https://doi.org/10.1111/tpj.14110
Leiva-Brondo M, Valcarcel M, Martí R, Roselló S, Cebolla-Cornejo J (2016) New opportunities for develo** tomato varieties with enhanced carotenoid content. Sci Agric 73:512–519. https://doi.org/10.1590/0103-9016-2015-0427
Liu H, Yu C, Li H, Ouyang B, Wang T, Zhang J, Wang X, Ye Z (2015) Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Sci 231:198–211. https://doi.org/10.1016/j.plantsci.2014.12.006
Liu Y, Huang W, **an Z, Hu N, Lin D, Ren H, Chen J, Su D, Li Z (2017) Overexpression of slgras40 in tomato enhances tolerance to abiotic stresses and influences auxin and gibberellin signaling. Front Plant Sci 8:1659. https://doi.org/10.3389/fpls.2017.01659
Lounsbery JK, Arms EM, Bloom AJ, Clair DA (2016) Quantitative trait loci for water-stress tolerance traits localize on chromosome 9 of wild tomato. Crop Sci 56:1514–1525. https://doi.org/10.2135/cropsci2015.07.0432
Lucini T, Faria MV, Rohde C, Resende JTV, Oliveira JRF (2015) Acylsugar and the role of trichomes in tomato genotypes resistance to Tetranychus urticae. Arthropod Plant Interact 9:45–53. https://doi.org/10.1007/s11829-014-9347-7
Martínez-Cuenca MR, Pereira-Dias L, Soler S, López-Serrano L, Alonso D, Calatayud Á, Díez MJ (2020) Adaptation to water and salt stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme. Agronomy 10:1169. https://doi.org/10.3390/agronomy10081169
Mata-Nicolás E, Montero-Pau J, Gimeno-Paez E, Garcia-Carpintero V, Ziarsolo P, Menda N, Mueller LA, Blanca J, Cañizares J, van der Knaap E, Díez MJ (2020) Exploiting the diversity of tomato: the development of a phenotypically and genetically detailed germplasm collection. Hortic Res 7:1–14. https://doi.org/10.1038/s41438-020-0291-7
Mbava N, Mutema M, Zengeni R, Shimelis H, Chaplot V (2020) Factors affecting crop water use efficiency: a worldwide meta-analysis. Agric Water Manag 228:105878. https://doi.org/10.1016/j.agwat.2019.105878
Millones-Chanamé CE, Oliveira AMS, Castro EM, Maluf WR (2019) Inheritance of blossom end rot resistance induced by drought stress and of associated stomatal densities in tomatoes. Euphytica 215:120. https://doi.org/10.1007/s10681-019-2444-z
Misra PK, Singh SN, Kumar P, Pandey MK (2019) Yield gap analysis, economics, adoption, and horizontal spread of tomato (Lycopersicon esculentum Mill) cultivation through front line demonstration in Eastern Uttar Pradesh, India. Int J Plant Environ 5:132–136. https://doi.org/10.18811/ijpen.v5i02.11
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55:1105–1113. https://doi.org/10.1093/jxb/erh113
Mohamed S, Ali E, Mohamed T (2012) Study of heritability and genetic variability among different plant and fruit characters of tomato (Solanum lycopersicum L.). IJSTR 1:55–58
Morales RG, Resende LV, Maluf WR, Peres LE, Bordini IC (2015) Selection of tomato plant families using characters related to water deficit resistance. Hortic Bras 33:27–33. https://doi.org/10.1590/S0102-053620150000100005
Moyle LC, Muir CD (2010) Reciprocal insights into adaptation from agricultural and evolutionary studies in tomato. Evol Appl 3:409–421. https://doi.org/10.1111/j.1752-4571.2010.00143.x
Mukherjee D, Maurya P, Bhattacharjee T, Banerjee S, Chatterjee S, Mal S, Chakraborty I, Maji A, Chattopadhyay A (2020) Assessment of breeding potential of cherry tomato [Solanum lycopersicum var. Cerasiforme (Dunnal) A. Gray] grown under open field to identify desirable alleles. Int J Curr Microbiol Appl Sci 9:2152–2171. https://doi.org/10.20546/ijcmas.2020.904.258
Nakazato T, Warren DL, Moyle LC (2010) Ecological and geographic modes of species divergence in wild tomatoes. Am J Bot 4:680–693. https://doi.org/10.3732/ajb.0900216
Nesbitt TC, Tanksley SD (2002) Comparative sequencing in the genus Lycopersicon. Implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162:365–379. https://doi.org/10.1093/genetics/162.1.365
Oliveira JRFd, Resende JTVd, Filho RBdL, Roberto SR, Silva PRd, Rech C, Nardi C (2020) Tomato breeding for sustainable crop systems: high levels of zingiberene providing resistance to multiple arthropods. Horticulturae 6:34. https://doi.org/10.3390/horticulturae6020034
Oliveira CS, Maciel GM, Fraga Júnior EF, Peixoto JVM, Assunção VB, Marques DJ (2021) Selection of tomato genotypes for drought tolerance and agronomic potential through different selection indexes. Hort Bras 39:102–111. https://doi.org/10.1590/s0102-0536-20210115
Pailles Y, Awlia M, Julkowska M, Passone L, Zemmouri K, Negrão S, Schmöckel SM, Tester M (2020) Diverse traits contribute to salinity tolerance of wild tomato seedlings from the Galapagos Islands. Plant Physiol 182:534–546. https://doi.org/10.1104/pp.19.00700
Peralta IE, Spooner DM (2000) Classification of wild tomatoes: a review. Kurtziana 28:45–54. http://hdl.handle.net/11336/152176
Peralta IE, Spooner DM, Knapp S (2008) Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst Bot Monogr 84:1–186
Petrović I, Savić S, Jovanović Z, Stikić R, Brunel B, Sérino S, Bertin N (2019) Fruit quality of cherry and large fruited tomato genotypes as influenced by water deficit. Zemdirb Agric 106:123–128. https://doi.org/10.13080/z-a.2019.106.016
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882. https://doi.org/10.1093/jxb/erq340
Ramírez-Ojeda G, Peralta IE, Rodríguez-Guzmán E, Chávez-Servia JL, Sahagún-Castellanos J, Rodríguez-Pérez JE (2021) Climatic diversity and ecological descriptors of wild tomato species (Solanum sect. lycopersicon) and close related species (Solanum sect. juglandifolia y sect. lycopersicoides) in Latin America. Plants 10:855. https://doi.org/10.3390/plants10050855
Ranc N, Muños S, Santoni S, Causse M (2008) A clarified position for solanum lycopersicum var. cerasiformein the evolutionary history of tomatoes (Solanaceae). BMC Plant Biol 8:130. https://doi.org/10.1186/1471-2229-8-130
Resende JT, Dias DM, Corte ED, Constantino LV, Ventura MU, de Lima Filho RB, de Oliveira LV, Da-Silva PR (2022) The introgression of resistance to Tuta absoluta in tomato based on glandular trichomes. Arthropod-Plant Interact 16:87–99. https://doi.org/10.1007/s11829-021-09873-x
Rick CM (1983) Genetic variability in tomato species. Plant Mol Biol Rep 1:81–87. https://doi.org/10.1007/BF02680303
Rigano MM, Arena C, Matteo AD, Sellitto S, Frusciante L, Barone A (2016) Eco-physiological response to water stress of drought-tolerant and drought-sensitive tomato genotypes. Plant Biosyst Int J Deal Asp Plant Biol 150:682–691. https://doi.org/10.1080/11263504.2014.989286
Rocha DK, Maciel GM, Fraga Junior EF, Machado Júnior CS, Nogueira GGS, Almeida RS (2016) Seleção de genótipos de tomateiro submetidos ao estresse hídrico em função da expressão de características fisiológicas. Rev Bras Ciênc Agrár 11:80–84. https://doi.org/10.5039/agraria.v11i2a5369
Santana MJ, Vieira TA (2010) Resposta do tomateiro irrigado a níveis de reposição de água no solo. Irriga 15:443–454. https://doi.org/10.15809/irriga.2010v15n4p443
She D, Sun X, Gamareldawla AHD, Nazar EA, Hu W, Edith K, Yu S (2018) Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci Rep 8:14743. https://doi.org/10.1038/s41598-018-33040-7
Shinwari A, Ahmad I, Khan I, Khattak H, Azimi AS (2018) Thermo-tolerance in tomato: acetyl salicylic acid affects growth and yield of tomato (Solanum Lycopersicum L.) under the agro-climatic condition of Islamabad, Pakistan. Adv Agric Environ Sci Open Access 1:102–107. https://doi.org/10.30881/aaeoa.00017
Silva RS, Kumar L, Shabani F, Picanço MC (2017) Assessing the impact of global warming on worldwide open field tomato cultivation through CSIRO-Mk3·0 global climate model. J Agric Sci 155:407–420. https://doi.org/10.1017/S0021859616000654
Silva AA, Carvalho RC, Andrade MC, Zeist AR, Resende JTV, Maluf WR (2019) Glandular trichomes that mediate resistance to green peach aphid in tomato genotypes from the cross between S. galapagense and S. lycopersicum. Acta Sci Agron 41:e42704–e42704. https://doi.org/10.4025/actasciagron.v41i1.42704
Solankey SS, Singh RK, Baranwal DK, Singh DK (2015) Genetic expression of tomato for heat and drought stress tolerance: an overview. Int J Veg Sci 21:496–515. https://doi.org/10.1080/19315260.2014.902414
Torrecillas A, Guillaume C, Alarcón JJ, Ruiz-Sánchez MC (1995) Water relations of two tomato species under water stress and recovery. Plant Sci 105:169–176. https://doi.org/10.1016/0168-9452(94)04048-6
Von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387. https://doi.org/10.1007/BF00384257
Yu D, Wildhagen H, Tylewicz S, Miskolczi PC, Bhalerao RP, Polle A (2019) Abscisic acid signalling mediates biomass trade-off and allocation in poplar. New Phytol 223:1192–1203. https://doi.org/10.1111/nph.15878
Zanin DS, Resende JTV, Zeist AR, Lima Filho RB, Gabriel A, Diniz FCP, Perrud AC, Morales RGF (2020) Selection of F2BC1 tomato genotypes for processing containing high levels of zingiberene and resistant to tomato pinworms. Phytoparasitica 49:265–274. https://doi.org/10.1007/s12600-020-00852-1
Zeist AR, Giacobbo CL, Silva Neto GF, Zeist RA, Dorneles KR, Resende JT (2018) Compatibility of tomato cultivar Santa Cruz Kada grafted on different Solanaceae species and control of bacterial wilt. Hortic Bras 36:377–381. https://doi.org/10.1590/s0102-053620180315
Zeist AR, Resende JT, Faria MV, Gabriel A, Adriano E, Lima Filho RB (2018) Photosynthetic characteristics in species and interspecific hybrids of tomato. Hortic Bras 36:362–370. https://doi.org/10.1590/s0102-053620180313
Zeist AR, Faria MV, Resende JTV, Gabriel A, Nonato JJ, Santos MH (2019) Biomass association in specimens and interspecific hybrids of tomatoes. Acta Sci Agron 42:e42806. https://doi.org/10.4025/actasciagron.v42i1.42806
Zeist AR, de Resende JT, Perrud AC, Gabriel A, Maluf WR, Arantes JH, Youssef K (2021) Resistance to Bemisia tabaci in tomato species and hybrids and its association with leaf trichomes. Euphytica 217:85. https://doi.org/10.1007/s10681-021-02815-x
Author information
Authors and Affiliations
Contributions
A.R.Z conceived the research idea; J.T.V.R contributed with discussions and made the plants available used in the experiments; A.C.P., A.D.S., J.N.M.O., and G.J.A.O conducted the experiment and collected the data; A.R.Z. and A.C.P did supervision; A.R.Z., J.M.H., A.C.P., and A.L.M analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeist, A.R., Henschel, J.M., Perrud, A.C. et al. Toward Drought Tolerance in Tomato: Selection of F2BC1 Plants Obtained from Crosses Between Wild and Commercial Genotypes. Agric Res 13, 26–40 (2024). https://doi.org/10.1007/s40003-023-00678-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-023-00678-3