Abstract
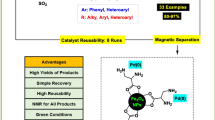
A novel adenine-based palladium nano-complex was synthesized via preparation of the Fe3O4 magnetic nano-particles (Fe3O4 MNPs), coating with tetraethyl orthosilicate, functionalization with both 3-chloropropyltrimethoxysilane (CPTMS) and adenine (AD) ligands and finally formation of the corresponding complex by PdCl2 (Fe3O4@SiO2@CPTMS@AD@ Pd). Then, it was characterized with different methods such as FT-IR, EDX, ICP, XRD, SEM, TEM, VSM and TGA-DTA techniques, and capability of the adenine-based Pd complex evaluated by its application as an efficient heterogeneous nano-catalyst for the synthesis of various N-mono-substituted ureas in water by hydrolysis of cyanamides in the presence of 98% HCOOH with good to high yields in relatively short reaction times at room temperature.












Similar content being viewed by others
References
J.L. Ochoa, J. Porath, J. Kempf, J.M. Egly, J. Chromatog. A. 188, 257 (1980)
E.J. Cho, B.J. Ryu, Y.J. Lee, K.C. Nam, Org. Lett. 7, 2607 (2005)
Y. Zhou, J.F. Zhang, J. Yoon, Chem. Rev. 114, 5511 (2014)
P.A. Gale, E.N. Howe, X. Wu, M.J. Spooner, Coord. Chem. Rev. 375, 333 (2018)
T.T. Bui, H.K. Kim, Synlett 31, 997 (2020)
M. Frezza, S. Castang, J. Estephane, L. Soulere, C. Deshayes, B. Chantegrel, Bioinorg. Med. Chem. 14, 4781 (2006)
A. Abad, J. Lloveras, A. Michelena, Field Crops Res. 87, 257 (2004)
S. Goyal, A. Chattopadhyay, K. Kasavajhala, U.D. Priyakumar, J. Am. Chem. Soc. 139, 14931 (2017)
S.J. Choi, J.H. Lee, Y.H. Lee, D.Y. Hwang, H.D. Kim, J. Appl. Polym. Sci. 121, 3516 (2011)
E.I. Pereira, F.B. Minussi, C.C. da Cruz, A.C. Bernardi, C. Ribeiro, J. Agric. Food Chem. 60, 5267 (2012)
V. Böhmer, M.O. Vysotsky, Aust. J. Chem. 54, 671 (2001)
L. Fischer, G. Guichard, Org. Biomol. Chem. 8, 3101 (2010)
C. Dou, C. Wang, H. Zhang, H. Gao, Y. Wang, Chem. Eur. J. 16, 10744 (2010)
M. Van Gool, J.M. Bartolomé, G.J. Macdonald, Tetrahedron Lett. 49, 7171 (2008)
I. Gallou, Org. Prep. Proceed. Int. 39, 355 (2007)
Q. Liu, N.W. Luedtke, Y. Tor, Tetrahedron Lett. 42, 1445 (2001)
E. Artuso, I. Degani, R. Fochi, C. Magistris, Synthesis 2007, 3497 (2007)
L. De Luca, A. Porcheddu, G. Giacomelli, I. Murgia, Synlett 2010, 2439 (2010)
S. Breitler, N.J. Oldenhuis, B.P. Fors, S.L. Buchwald, Org. lett. 13, 3262 (2011)
S.M. Sajadi, M. Maham, J. Chem. Res. 37, 623 (2013)
H. Mahajan, M. Bhardwaj, S. Paul, Org. Prep. Proceed. Int. 46, 463 (2014)
A.R. Sardarian, I.D. Inaloo, RSC Adv. 5, 76626 (2015)
L. Wang, H. Wang, G. Li, S. Min, F. **ang, S. Liu, W. Zheng, Adv. Synth. Catal. 360, 4585 (2018)
I.D. Inaloo, S. Majnooni, M. Esmaeilpour, Eur. J. Org. Chem. 26, 3481 (2018)
D. Habibi, S. Heydari, A. Faraji, H. Keypour, M. Mahmoudabadi, Polyhedron 151, 520 (2018)
J.A. Grzyb, M. Shen, C. Yoshina-Ishii, W. Chi, R.S. Brown, R.A. Batey, Tetrahedron 61, 7153 (2005)
G. Meng, M. Wang, M.S. Dong, A.Q. Zheng, J. Shi, E. De Clercq, C. Pannecouque, J. Balzarini, Int. J. AIDS Res. 2, 19 (2015)
K. Somakala, M. Amir, Acta Pharmaceut. Sinica B 7, 230 (2017)
A.R. Kulkarni, S. Garai, G.A. Thakur, J. Org. Chem. 82, 992 (2017)
M. Xu, A.R. Jupp, M.S. Ong, K.I. Burton, S.S. Chitnis, D.W. Stephan, Angew. Chem. Int. Ed. 58, 5707 (2019)
V. Khorramabadi, D. Habibi, S. Heydari, Green Chem. Lett. Rev. 13, 50 (2020)
N. Amirmahani, N.O. Mahmoodi, M. Malakootian, A. Pardakhty, N. Seyedi, Mater. Chem. Phys. 267, 124698 (2021)
M. Mozaffari, N. Nowrouzi, Eur. J. Org. Chem. 46, 7541 (2019)
D.K. Yadav, A.K. Yadav, V.P. Srivastava, G. Watal, L.D.S. Yadav, Tetrahedron Lett. 53, 2890 (2012)
H.K.A. Kim, Org. Biomol. Chem. 14, 7345 (2016)
M. Patil, A.N. Poyil, S.D. Joshi, S.A. Patil, S.A. Patil, A. Bugarin, Bioorg. Chem. 87, 302 (2019)
E.V. Vinogradova, B.P. Fors, S.L. Buchwald, J. Am. Chem. Soc. 134, 11132 (2012)
Acknowledgements
We thank Bu-Ali Sina University, Hamedan Iran, for the fiscal support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khorram Abadi, V., Habibi, D., Heydari, S. et al. An adenine-based palladium complex: a capable heterogeneous magnetic nano-catalyst for the green synthesis of the ureas in aqueous media. J IRAN CHEM SOC 20, 1985–1996 (2023). https://doi.org/10.1007/s13738-023-02813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02813-x