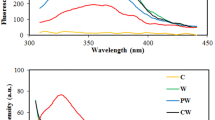
Abstract
Curcumin is a natural polyphenol with various bioactivities, including anti-tumor, antioxidant, and anti-inflammatory. Despite curcumin’s therapeutic benefits, limited water solubility and rapid hydrolytic degradation are important challenges in using of curcumin for clinical applications. Therefore, this report aimed to examine the stability and antioxidant activity of curcumin in different environments and to find out the mechanism of action through which curcumin shows its radical scavenging activity. Results of this study not only indicated that degradation of curcumin is water-dependent but also suggested its degradation mechanism is distinct from general acid/base catalysis and nucleophilic attack of water hydroxyl ion (OH−) had apparently negligible role in rapid hydrolytic degradation of curcumin. We confirmed that curcumin is solely responsible for its antioxidant activity in aqueous media and its degradation is a spontaneous autoxidation. More importantly, curcumin exhibited rapid decomposition in the presence of excessive O2 or free radicals induced by oxidizing agents (TBHP and H2O2) due to its protective potential against oxidative stress. The possible attack site of free radicals in curcumin is in the first step of curcumin degradation, where phenoxyl radical is produced. On the other hand, curcumin degradation and scavenging activity reduced when it entered into the hydrophobic cavity of BSA or casein molecule. Surprisingly, in soybean oil, curcumin showed the weak antioxidant activity compared to TBHQ (as a potent synthetic antioxidant for frying oils), implying that curcumin did not have significant antioxidant potential in oily/hydrophobic environments. Overall, our findings confirmed that curcumin in aqueous buffer is quickly degrades while at the same time scavenging of free radicals. The presented results are novel and might be interesting as the analysis of physicochemistry of curcumin has been gaining importance in view of biological significance.













Similar content being viewed by others
References
M.M. Nadi, M.R. Ashrafi Kooshk, K. Mansouri, S.A. Ghadami, M. Amani, S. Ghobadi, R. Khodarahmi, Int. J. Food Prop. 18, 638–659 (2015)
C.-H. Hsu, A.-L. Cheng, The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease (Springer, Berlin, 2007), pp. 471–480
M. Mehrabi, S. Esmaeili, M. Ezati, M. Abassi, H. Rasouli, D. Nazari, H. Adibi, R. Khodarahmi, Bioorg. Chem. 110, 104720 (2021)
R. Sharma, A. Gescher, W. Steward, Eur. J. Cancer. 41, 1955–1968 (2005)
C.F. Chignell, P. Bilskj, K.J. Reszka, A.G. Motten, R.H. Sik, T.A. Dahl, Photochem. Photobiol. 59, 295–302 (1994)
T. Ak, İ Gülçin, Chem. Biol. Interact. 174, 27–37 (2008)
O.N. Gordon, P.B. Luis, H.O. Sintim, C. Schneider, J. Biol. Chem. 290, 4817–4828 (2015)
M. Griesser, V. Pistis, T. Suzuki, N. Tejera, D.A. Pratt, C. Schneider, J. Biol. Chem. 286, 1114–1124 (2011)
A. Kumar, A. Ahuja, J. Ali, S. Baboota, Crit. Rev. Ther. Drug Carrier Syst. 27, 279–312 (2010)
Y.-J. Wang, M.-H. Pan, A.-L. Cheng, L.-I. Lin, Y.-S. Ho, C.-Y. Hsieh, J.-K. Lin, J. Pharm. Biomed. Anal. 15, 1867–1876 (1997)
C. Schneider, O.N. Gordon, R.L. Edwards, P.B. Luis, J. Agric. Food Chem. 63, 7606–7614 (2015)
O.N. Gordon, C. Schneider, Trends Mol. Med. 18, 361–363 (2012)
J. Zhu, K.Z. Sanidad, E. Sukamtoh, G. Zhang, Food Funct. 8, 907–914 (2017)
L. Shen, C.-C. Liu, C.-Y. An, H.-F. Ji, Sci. Rep. 6, 1–10 (2016)
J. Jankun, M. Wyganowska-Świątkowska, K. Dettlaff, A. Jelińska, A. Surdacka, D. Wątróbska-Świetlikowska, E. Skrzypczak-Jankun, Int. J. Mol. Med. 37, 1151–1158 (2016)
A. Hunyadi, Med. Res. Rev. 39, 2505–2533 (2019)
C. Evans, G. List, H.A. Moser, J.J. Cowan, J. Am. Oil Chem. Soc. 50, 218–222 (1973)
L. Brühl, Wiley Online Library (AOCS Press, Champaign, 1997)
M.S. Blois, Nature 181, 1199–1200 (1958)
M. Burits, F. Bucar, Phytother. Res. 14, 323–328 (2000)
I. Gülçin, Amino Acids 32, 431–438 (2007)
J. Ancerewicz, E. Migliavacca, P.-A. Carrupt, B. Testa, F. Brée, R. Zini, J.-P. Tillement, S. Labidalle, D. Guyot, A.-M. Chauvet-Monges, Free Radic. Biol. Med. 25, 113–120 (1998)
Y. Nimiya, W. Wang, Z. Du, E. Sukamtoh, J. Zhu, E. Decker, G. Zhang, Mol. Nutr. Food Res. 60, 487–494 (2016)
D. Voet, J.G. Voet, Biochemistry (Wiley, New York, 1995), pp. 506–516
H. Sies, C. Berndt, D.P. Jones, Annu. Rev. Biochem. 86, 715–748 (2017)
T. Kar, P. Basak, S. Sen, R.K. Ghosh, M. Bhattacharyya, Front. Biol. 12, 199–209 (2017)
F. Zsila, Z. Bikádi, M.J.B. Simonyi, Biochem. Biophys. Res. Commun. 301, 776–782 (2003)
B. Pastrello, G.C. dos Santos, L.C. da Silva-Filho, A.R. de Souza, N.H. Morgon, V.F. **menes, Dyes Pigm. 173, 107874 (2020)
O.A. Kucherak, L. Richert, Y. Mély, A.S. Klymchenko, Phys. Chem. Chem. Phys. 14, 2292–2300 (2012)
S. Ercelen, A.S. Klymchenko, A.P. Demchenko, FEBS Lett. 538, 25–28 (2003)
M. Yang, Y. Wu, J. Li, H. Zhou, X.J. Wang, Agric. Food Chem. 61, 7150–7155 (2013)
F. Mirzaee, L. Hosseinzadeh, M.R. Ashrafi-Kooshk, S. Esmaeili, S. Ghobadi, M.H. Farzaei, M.R. Zad-Bari, R. Khodarahmi, Protein Pept. Lett. 26, 132–147 (2019)
M.A.D. Guillén, N. Cabo, Food Chem. 77, 503–510 (2002)
A.H. Sneharani, S.A. Singh, A.J. Appu Rao, J. Agric. Food Chem. 57, 10386–10391 (2009)
P. Bharmoria, M. Bisht, M.C. Gomes, M. Martins, M.C. Neves, J.F. Mano, I. Bdikin, J.A. Coutinho, S.P. Ventura, Sci. Rep. 11, 1–12 (2021)
S. Rege, M. Arya, S. Momin, Ukr. J. Food Sci. 7, 27–32 (2019)
S.A. Rege, M.A. Varshneya, S.A.J.C. Momin, J. Food Sci. Technol. 13, 128–132 (2021)
Acknowledgements
The authors gratefully acknowledge the Student Research committee, Kermanshah University of Medical sciences (KUMS), Kermanshah, Iran, for the financial supports (Grant no. 50001082).
Author information
Authors and Affiliations
Contributions
RK designed the study. MS, FK and SE performed experiments/assays under supervision of RK and MM All authors, especially, MM and RK contributed to the writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Rights and permissions
About this article
Cite this article
Mehrabi, M., Karami, F., Siah, M. et al. Is curcumin an active suicidal antioxidant only in the aqueous environments?. J IRAN CHEM SOC 19, 3441–3450 (2022). https://doi.org/10.1007/s13738-022-02538-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02538-3