Abstract
Abstract
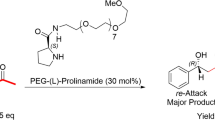
Synthesis of prolinamide derivatives of (R)-tetrahydropapaverine as mono-, di- and tripeptide is reported. (R)-Tetrahydropapaverine-prolinamide hybrid derivatives were tested as organocatalysts in the asymmetric Aldol reaction of aldehydes and ketones within various solvents, temperatures, and molar ratios. Catalyst 2 (30 mol %) afforded the best result in the Aldol reaction of cyclohexanone with 4-nitrobenzaldehyde up to 90% ee. Although in the sole presence of l-proline the reaction proceeded with anti-stereoselectivity, its hybridization with (R)-tetrahydropapaverine yielded the formation of syn products as the major compounds.
Graphical abstract






Similar content being viewed by others
References
Nobel Prize Outreach AB 2021 (2021), https://www.nobelprize.org/prizes/chemistry/
S. Bahmanyar, K.N. Houk, J. Am. Chem. Soc. 123, 12911 (2001)
B. List, R.A. Lerner, C.F. Barbas, J. Am. Chem. Soc. 122, 2395 (2000)
B. List, R.A. Lerner, C.F. Barbas, Org. Lett. 1, 59 (1999)
M.T. Robak, M.A. Herbage, J.A. Ellman, Chem. Rev. 110, 3600 (2010)
I.R. Shaikh, J. Catal. 2014, 1 (2014)
S. Zhang, X. Fu, S. Fu, Tetrahedron Lett. 50, 1173 (2009)
J. Paradowska, M. Rogozińska, J. Mlynarski, Tetrahedron Lett. 50, 1639 (2009)
Z. Tang, Z.-H. Yang, X.-H. Chen, L.-F. Cun, A.-Q. Mi, Y.-Z. Jiang, L.-Z. Gong, J. Am. Chem. Soc. 127, 9285 (2005)
W. Li, J. Wang, X. Hu, K. Shen, W. Wang, Y. Chu, L. Lin, X. Liu, X. Feng, J. Am. Chem. Soc. 132, 8532 (2010)
J.G. Hernandez, E. Juaristi, J. Org. Chem. 76, 1464 (2011)
H. Yang, S. Mahapatra, P.H.-Y. Cheong, R.G. Carter, J. Org. Chem. 75, 7279 (2010)
J.-R. Chen, X.-L. An, X.-Y. Zhu, X.-F. Wang, W.-J. **ao, J. Org. Chem. 73, 6006 (2008)
H. Wynberg, Top. Stereochem. 16, 87 (1986)
M. Dabiri, P. Salehi, G. Kozehgary, S. Heydari, A. Heydari, M. Esfandyari, Tetrahedron Asymmetry 19, 1970 (2008)
M. Mohebbi, P. Salehi, M. Bararjanian, S.N. Ebrahimi, J. Iran. Chem. Soc. 15, 47 (2018)
M. Mohebbi, M. Bararjanian, S.N. Ebrahimi, M. Smieško, P. Salehi, Synthesis 50, 1841 (2018)
L.F. Tietze, H.P. Bell, S. Chandrasekhar, Angew. Chem. Int. Ed. 42, 3996 (2003)
G.D. Yadav, Deepa, S. Singh, ChemistrySelect 4, 5591 (2019)
C. Wang, Y. Jiang, X. Zhang, Y. Huang, B. Li, G. Z.-T. letters, and undefined 2007, Elsevier (2007)
E. Machuca, E. Juaristi, Tetrahedron Lett. 56, 1144 (2015)
J.G. Hernández, E. Juaristi, Tetrahedron 67, 6953 (2011)
J.G. Hernández, E. Juaristi, J. Org. Chem. 76, 1464 (2011)
R. Thiyagarajan, Z. Begum, C. Seki, Y. Okuyama, E. Kwon, K. Uwai, M. Tokiwa, S. Tokiwa, M. Takeshita, H. Nakano, RSC Adv. 11, 38925 (2021)
I. Vlasserou, M. Sfetsa, D.T. Gerokonstantis, C.G. Kokotos, P. Moutevelis-Minakakis, Tetrahedron 74, 2338 (2018)
H. Akutsu, K. Nakashima, H. Yanai, A. Kotani, S.I. Hirashima, T. Yamamoto, R. Takahashi, A. Yoshida, Y. Koseki, H. Hakamata, T. Matsumoto, T. Miura, Synlett 28, 1363 (2017)
C. Wang, Y. Jiang, X. **a-Zhang, Y. Huang, B. Gang-Li, G. Lin-Zhang, Tetrahedron Lett. 48, 4281 (2007)
D.D. Chronopoulos, M. Tsakos, N. Karousis, C.G. Kokotos, N. Tagmatarchis, Mater. Lett. 137, 343 (2014)
B. Rodríguez, T. Rantanen, C. Bolm, Angew. Chem. 118, 7078 (2006)
Acknowledgements
We are thankful to Shahid Beheshti University Research Council for partial support of this work. Also cooperation of Tofigh Daru Research and Engineering Company in this project is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
13738_2022_2535_MOESM1_ESM.pdf
Supporting information for this article is available on the WWW under http://dx.doi.org/ (PDF 1298 kb)
Rights and permissions
About this article
Cite this article
Naeimi, S.S., Salehi, P. & Bararjanian, M. New organocatalysts derived from tetrahydropapaverine for asymmetric aldol reaction. J IRAN CHEM SOC 19, 3407–3416 (2022). https://doi.org/10.1007/s13738-022-02535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02535-6