Abstract
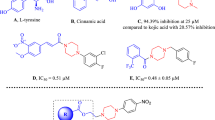
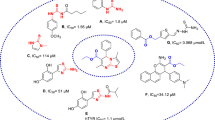
In this study, a series of new organic compounds with piperazine as a fundamental skeleton was synthesized and evaluated for their tyrosinase inhibitory potentials by in vitro and in silico studies. The in vitro studies have shown that compounds 10a and 10b bearing 1,2,4, triazole nucleus could be considered potent tyrosinase inhibitors with IC50 values of 31.2 ± 0.7 and 30.7 ± 0.2 µM, respectively. 10b (Ki = 9.54 µM, mixed type inhibition) with the lowest IC50 value among derivatives was selected to determine kinetic constants and inhibition types. Furthermore, molecular docking analysis was performed for all compounds and it was observed that 4b, 5a, 4c, and 10b showed promising inhibitory effect on tyrosinase activity. Based on docking results, ADME predictions and in vitro studies, 10b might be considered suitable oral drug candidates for further studies.




Similar content being viewed by others
References
C.S. Nunes, K. Vogel, in Enzymes, in Human and Animal Nutrition. ed. by C.S. Nunes, V. Kumar (Elsevier, Academic Press, 2018), pp. 403–412
Á. Sánchez-Ferrer, J. Neptuno Rodríguez-López, F. García-Cánovas, F. García-Carmona, Biochim. Biophys. Acta, 1247(1), 1–11 (1995). https://doi.org/10.1016/0167-4838(94)00204-T
G. Prota, Med. Res. Rev. 8(4), 525–556 (1988). https://doi.org/10.1002/med.2610080405
H. Ando, H. Kondoh, M. Ichihashi, V.J. Hearing, J. Invest. Dermatol. 127(4), 751–761 (2007). https://doi.org/10.1038/sj.jid.5700683
L.L. Baxter, W.J. Pavan, Wiley Interdiscip. Rev. Dev. Biol. 2(3), 379–392 (2013). https://doi.org/10.1002/wdev.72
T. Hasegawa, Int. J. Mol. Sci. 11(3), 1082–1089 (2010). https://doi.org/10.3390/ijms11031082
T. Pillaiyar, M. Manickam, V. Namasivayam, J. Enzyme Inhib. Med. Chem. 32(1), 403–425 (2017). https://doi.org/10.1080/14756366.2016.1256882
S. Briganti, E. Camera, M. Picardo, Pigment Cell Res. 16(2), 101–110 (2003). https://doi.org/10.1034/j.1600-0749.2003.00029.x
L. Ni-Komatsu, C. Tong, G. Chen, N. Brindzei, S.J. Orlow, Mol. Pharmacol. 74(6), 1576–1586 (2008). https://doi.org/10.1124/mol.108.050633
R.F. Hurrell, P.-A. Finot, Adv. Exp. Med. Biol. 177, 423–435 (1984). https://doi.org/10.1007/978-1-4684-4790-3_20
M.R. Loizzo, R. Tundis, F. Menichini, Compr. Rev. Food Sci. F. 11(4), 378–398 (2012). https://doi.org/10.1111/j.1541-4337.2012.00191.x
S. Parvez, M. Kang, H.-S. Chung, H. Bae, Phytother. Res. 21(9), 805–816 (2007). https://doi.org/10.1002/ptr.2184
T. Damghani, S. Hadaegh, M. Khoshneviszadeh, S. Pirhadi, R. Sabet, M. Khoshneviszadeh, N. Edraki, J. Mol. Struct. 1222, 128876–128876 (2020). https://doi.org/10.1016/j.molstruc.2020.128876
D. Yang, L. Wang, J. Zhai, N. Han, Z. Liu, S. Li, J. Yin, Food Chem. 336, 127714–127714 (2021). https://doi.org/10.1016/j.foodchem.2020.127714
H. Hosseinpoor, A. Iraji, N. Edraki, S. Pirhadi, M. Attarroshan, M. Khoshneviszadeh, M. Khoshneviszadeh, Chem. Biodivers. 17(8), e2000285 (2020). https://doi.org/10.1002/cbdv.202000285
H. Raza, M. A. Abbasi, R. Aziz ur, S. Z. Siddiqui, M. Hassan, Q. Abbas, H. Hong, S. A. A. Shah, M. Shahid, S. Y. Seo, Bioorg. Chem., 94, 103445–103445 (2020). https://doi.org/10.1016/j.bioorg.2019.103445
S.H. Shelke, P.C. Mhaske, S.K. Kasam, V.D. Bobade, J. Heterocycl. Chem. 51(6), 1893–1897 (2014). https://doi.org/10.1002/jhet.1910
Z. Shi, Z. Zhao, M. Huang, X. Fu, C. R. Chim. 18(12), 1320–1327 (2015). https://doi.org/10.1016/j.crci.2015.09.005
S.H. Shelke, P.C. Mhaske, S. Narkhade, V.D. Bobade, J. Heterocycl. Chem. 51(4), 1151–1156 (2014). https://doi.org/10.1002/jhet.1789
R. Listro, S. Stotani, G. Rossino, M. Rui, A. Malacrida, G. Cavaletti, M. Cortesi, C. Arienti, A. Tesei, D. Rossi, M.D. Giacomo, M. Miloso, S. Collina, Front. Chem. 8, 495–495 (2020). https://doi.org/10.3389/fchem.2020.00495
G.D. Hatnapure, A.P. Keche, A.H. Rodge, S.S. Birajdar, R.H. Tale, V.M. Kamble, Bioorg. Med. Chem. Lett. 22(20), 6385–6390 (2012). https://doi.org/10.1016/j.bmcl.2012.08.071
B. Selvakumar, N. Gujjar, M. Subbiah, K.P. Elango, Med. Chem. Res. 27(2), 512–519 (2018). https://doi.org/10.1007/s00044-017-2077-5
J.A. Wiles, B.J. Bradbury, M.J. Pucci, Expert Opin. Ther. Pat. 20(10), 1295–1319 (2010). https://doi.org/10.1517/13543776.2010.505922
M. Baumann, I.R. Baxendale, Beilstein J. Org. Chem. 9(1), 2265–2319 (2013). https://doi.org/10.3762/bjoc.9.265
S.J.Y. Macalino, V. Gosu, S. Hong, S. Choi, Arch. Pharm. Res. 38(9), 1686–1701 (2015). https://doi.org/10.1007/s12272-015-0640-5
X. Meng, H.-X. Zhang, M. Mezei, M. Cui, Curr. Comput. Aided-Drug 7(2), 146–157 (2011). https://doi.org/10.2174/157340911795677602
S. Başoğlu Özdemir, J. Turkish Chem. Soc., 3(3), 515–534 (2016). https://doi.org/10.18596/jotcsa.55734
M.Y. Mentese, H. Bayrak, Y. Uygun, A. Mermer, S. Ulker, S.A. Karaoglu, N. Demirbas, Eur. J. Med. Chem. 67, 230–242 (2013). https://doi.org/10.1016/j.ejmech.2013.06.045
E. Rajanarendar, K. Thirupathaiah, S. Ramakrishna, D. Nagaraju, Chin. Chem. Lett. 26(12), 1511–1513 (2015). https://doi.org/10.1016/j.cclet.2015.07.024
A. Balabani, D.J. Hadjipavlou-Litina, K.E. Litinas, M. Mainou, C.-C. Tsironi, A. Vronteli, Eur. J. Med. Chem. 46(12), 5894–5901 (2011). https://doi.org/10.1016/j.ejmech.2011.09.053
H. Bayrak, A. Demirbas, N. Demirbas, S.A. Karaoglu, Eur. J. Med. Chem. 44(11), 4362–4366 (2009). https://doi.org/10.1016/j.ejmech.2009.05.022
Z. Peng, G. Wang, Q.-H. Zeng, Y. Li, Y. Wu, H. Liu, J.J. Wang, Y. Zhao, Food Chem. 341, 128265–128265 (2021). https://doi.org/10.1016/j.foodchem.2020.128265
B.D. Vanjare, P.G. Mahajan, N.C. Dige, H. Raza, M. Hassan, Y. Han, S.J. Kim, S.-Y. Seo, K.H. Lee, Mol. Divers. (2020). https://doi.org/10.1007/s11030-020-10102-5
U. Cakmak, F. Oz-Tuncay, S. Basoglu-Ozdemir, E. Ayazoglu-Demir, İ Demir, A. Colak, S. Celik-Uzuner, S.S. Erdem, N. Yildirim, Med. Chem. Res. 30(10), 1886–1904 (2021). https://doi.org/10.1007/s00044-021-02785-8
C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Adv. Drug Del. Rev. 23(1–3), 3–25 (1997). https://doi.org/10.1016/S0169-409X(96)00423-1
D.F. Veber, S.R. Johnson, H.-Y. Cheng, B.R. Smith, K.W. Ward, K.D. Kopple, J. Med. Chem. 45(12), 2615–2623 (2002). https://doi.org/10.1021/jm020017n
W. T. Ismaya, H. t. J. Rozeboom, A. Weijn, J. J. Mes, F. Fusetti, H. J. Wichers, B. W. Dijkstra, Biochemistry, 50(24), 5477–5486 (2011). https://doi.org/10.1021/bi200395t
S. Jolivet, N. Arpin, H.J. Wichers, G. Pellon, Mycol. Res. 102(12), 1459–1483 (1998). https://doi.org/10.1017/S0953756298006248
L. Gou, Z.-R. Lü, D. Park, S.H. Oh, L. Shi, S.J. Park, J. Bhak, Y.-D. Park, Z.-L. Ren, F. Zou, J. Biomol. Struct. Dyn. 26(3), 395–401 (2008). https://doi.org/10.1080/07391102.2008.10507254
J.C. Espin, M. Morales, R. Varon, J. Tudela, F. Garciacanovas, Anal. Biochem. 231(1), 237–246 (1995). https://doi.org/10.1006/abio.1995.1526
Y. Ozdemir, O. Bekircan, A. Colak, C. Dokuzparmak, Indian J. Chem., 59, 1409–1417 (2020). http://nopr.niscair.res.in/handle/123456789/55444
Y. Kolcuoğlu, A. Colak, E. Sesli, M. Yildirim, N. Saglam, Food Chem. 101(2), 778–785 (2007). https://doi.org/10.1016/j.foodchem.2006.02.035
C. Molinspiration (2016). http://www.molinspiration.com/cgi-bin/properties
Y.H. Zhao, M.H. Abraham, J. Le, A. Hersey, C.N. Luscombe, G. Beck, B. Sherborne, I. Cooper, Pharm. Res. 19(10), 1446–1457 (2002). https://doi.org/10.1023/A:1020444330011
S. Wavefunction (2016). http://wavefun.com
J.J.P. Stewart, J. Mol. Model. 15(7), 765–805 (2009). https://doi.org/10.1007/s00894-008-0420-y
Y. Zhao, D.G. Truhlar, Acc. Chem. Res. 41(2), 157–167 (2008). https://doi.org/10.1021/ar700111a
Y. Zhao, D.G. Truhlar, Theor. Chem. Acc. 120(1–3), 215–241 (2008). https://doi.org/10.1007/s00214-007-0310-x
O. Trott, A.J. Olson, J. Comput. Chem. 31(2), 455–461 (2009). https://doi.org/10.1002/jcc.21334
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, A.J. Olson, J. Comput. Chem. 30(16), 2785–2791 (2009). https://doi.org/10.1002/jcc.21256
Accelrys Software Inc. Release 4.0, San Diego, Discovery Studio Modeling Environment (2013).
Acknowledgements
This work was supported by the Scientific and Research Council of Turkey (TUBITAK) [No. 117Z199].
Funding
This work was supported by the Scientific and Research Council of Turkey (TUBITAK) [No. 117Z199], which is hereby gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dokuzparmak, C., Oz Tuncay, F., Basoglu Ozdemir, S. et al. Newly synthesized piperazine derivatives as tyrosinase inhibitors: in vitro and in silico studies. J IRAN CHEM SOC 19, 2739–2748 (2022). https://doi.org/10.1007/s13738-021-02487-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02487-3