Abstract
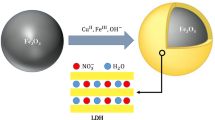
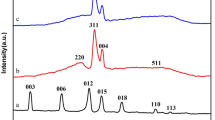
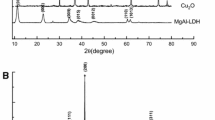
Magnetic CuFe-layered double hydroxide (Fe3O4@CuFe-LDH) nanocomposites were prepared with different Cu/Fe molar ratios for boosting the persulfate (Ps) activation under visible light irradiation. The LDH products were characterized in different ways. Recalcitrant quinoline (Qu) was the target pollutant in the experiments. It was found that the presence of the LDH photocatalyst caused significant enhancement in the Qu degradation so that the Vis/Ps/Fe3O4@LDH process could give 92.1% degradation and 70.5% mineralization efficiencies after 30 min operations at room temperature. The appropriate optimum conditions were Ps concentration of 450 mg/L, catalyst dosage of only 35 mg/L and the Cu/Fe molar ratio in the LDH structure of 3:1. The solution natural pH of about 6 provided the best performance. The LDH photocatalyst regeneration, in five cycles, each after a simple elution, exhibited good stability and reusability with an overall decrease of 4.9% in the activity. The presence of some co-existing anions of aqueous solutions causes diminishing the process efficiency. A pathway of Qu mineralization was proposed based on the identified intermediates. Taking into account the electrical energy and the consumed materials, an operating cost of $31.6/m3 was estimated for one order of magnitude reduction in the pollutant concentration.











Similar content being viewed by others
References
M. Kermani, F. Mohammadi, B. Kakavandi, A. Esrafili, Z. Rostamifasih, Simultaneous catalytic degradation of 2, 4-D and MCPA herbicides using sulfate radical-based heterogeneous oxidation over persulfate activated by natural hematite (α-Fe2O3/PS). J. Phys. Chem. Solids 117, 49–59 (2018)
Q. Ma, L.C. Nengzi, B. Li, Z. Wang, L. Liu, X. Cheng, Heterogeneously catalyzed persulfate with activated carbon coated with CoFe layered double hydroxide (AC@CoFe-LDH) for the degradation of lomefloxacin. Sep. Purif. Technol. 235, 116204 (2020)
T.K. Lau, W. Chu, N.J. Graham, The aqueous degradation of butylated hydroxyanisole by UV/S2O82-: study of reaction mechanisms via dimerization and mineralization. Environ. Sci. Technol. 41, 613–619 (2007)
C. Gong, F. Chen, Q. Yang, K. Luo, F. Yao, S. Wang, X. Wang, J. Wu, X. Li, D. Wang, Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 321, 222–232 (2017)
M. Mahdi-Ahmed, S. Chiron, Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater. J. Hazard. Mater. 265, 41–46 (2014)
Y. Ma, F. Chen, Q. Yang, Y. Zhong, X. Shu, F. Yao, T. **e, X. Li, D. Wang, G. Zeng, Sulfate radical induced degradation of Methyl Violet azo dye with CuFe layered doubled hydroxide as heterogeneous photoactivator of persulfate. J. Environ. Manage. 227, 406–414 (2018)
L. Wang, Q. Gao, Z. Li, Y. Wang, Improved removal of quinoline from wastewater using coke powder with inorganic ions. Processes 8, 156 (2020)
M. Ghasemi, A. Khataee, P. Gholami, R.D.C. Soltani, Template-free microspheres decorated with Cu-Fe-NLDH for catalytic removal of gentamicin in heterogeneous electro-Fenton process. J. Environ. Manage 248, 109236 (2019)
C. Wang, K. Ma, T. Wu, M. Ye, P. Tan, K. Yan, Electrochemical mineralization pathway of quinoline by boron-doped diamond anodes. Chemosphere 149, 219–223 (2016)
G. Zhang, X. Zhang, Y. Meng, G. Pan, Z. Ni, S. **a, Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: a review. Chem. Eng. J 392, 123684 (2020)
K. Padoley, S. Mudliar, R. Pandey, Heterocyclic nitrogenous pollutants in the environment and their treatment options–an overview. Bioresource Technol. 99, 4029–4043 (2008)
N.J. Pachupate, P.D. Vaidya, Catalytic wet oxidation of quinoline over Ru/C catalyst. J. Environ. Chem. Eng. 6, 883–889 (2018)
H. Zhu, W. Ma, H. Han, Y. Han, W. Ma, Catalytic ozonation of quinoline using nano-MgO: Efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 327, 91–99 (2017)
J. **g, W. Li, A. Boyd, Y. Zhang, V.L. Colvin, W.Y. William, Photocatalytic degradation of quinoline in aqueous TiO2 suspension. J. Hazard. Mater. 237, 247–255 (2012)
S. Pandeya, A. Tyagi, Synthetic approaches for quinoline and isoquinoline. ChemInform 43, 53–61 (2012)
L.P. Ramírez, K. Landfester, Magnetic polystyrene nanoparticles with a high magnetite content obtained by miniemulsion processes. Macromol. Chem. Phys. 204, 22–31 (2003)
H.R. Mardani, (Cu/Ni)–Al layered double hydroxides@ Fe3O4 as efficient magnetic nanocomposite photocatalyst for visible-light degradation of methylene blue. Res. Chem. Intermediat. 43, 5795–5810 (2017)
Z. Mesgari, J. Saien, Pollutant degradation over dye sensitized nitrogen doped titania substances in different configurations of visible light helical flow photoreactor. Sep. Purif. Technol. 185, 129–139 (2017)
A. Nedoloujko, J. Kiwi, Parameters affecting the homogeneous and heterogeneous degradation of quinoline solutions in light-activated processes. J. Photochem. Photobiol. 110, 149–157 (1997)
J. Saien, H. Shafiei, A. Amisama, Photo-activated periodate in homogeneous degradation and mineralization of quinoline: Optimization, kinetic, and energy consumption. Environ. Prog. Sustain. 36, 1621–1627 (2017)
L. Lu, J. Li, D.H. Ng, P. Yang, P. Song, M. Zuo, Synthesis of novel hierarchically porous Fe3O4@ MgAl–LDH magnetic microspheres and its superb adsorption properties of dye from water. J. Ind. Eng. Chem. 46, 315–323 (2017)
L. Adlnasab, M. Ezoddin, M.A. Karimi, N. Hatamikia, MCM-41@Cu–Fe–LDH magnetic nanoparticles modified with cationic surfactant for removal of Alizarin Yellow from water samples and its determination with HPLC. Res. Chem. Intermediat. 44, 3249–3265 (2018)
H. Wang, M. **g, Y. Wu, W. Chen, Y. Ran, Effective degradation of phenol via Fenton reaction over CuNiFe layered double hydroxides. J. Hazard. Mater. 353, 53–61 (2018)
D. Dumbre, V.R. Choudhary, P. Selvakannan, Cu–Fe layered double hydroxide derived mixed metal oxide: environmentally benign catalyst for Ullmann coupling of aryl halides. Polyhedron 120, 180–184 (2016)
J. Yan, Y. Chen, L. Qian, W. Gao, D. Ouyang, M. Chen, Heterogeneously catalyzed persulfate with a CuMgFe layered double hydroxide for the degradation of ethylbenzene. J. Hazard. Mater. 338, 372–380 (2017)
N. Baliarsingh, L. Mohapatra, K. Parida, Design and development of a visible light harvesting Ni–Zn/Cr–CO32− LDH system for hydrogen evolution. J. Mater. Chem. 1, 4236–4243 (2013)
H. Hori, A. Yamamoto, E. Hayakawa, S. Taniyasu, N. Yamashita, S. Kutsuna, H. Kiatagawa, R. Arakawa, Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 39, 2383–2388 (2005)
J. Saien, S. Seyyedan, High performance homogeneous photo-activated persulfate for nicotinic acid removal, intensified with copper ions and ultrasonic waves. Process. Saf. Environ. Prot. 131, 300–306 (2019)
M. Zulfakar, N. Hairul, H. Akmal, M.A. Rahman, Photocatalytic degradation of phenol in a fluidized bed reactor utilizing immobilized TiO2 photocatalyst: characterization and process studies. J. Appl. Sci. 11, 2320–2326 (2011)
X. Gao, C. Maa, Y. Liua, L. **ng, Y. Yan, Self-induced Fenton reaction constructed by Fe(III) grafted BiVO4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Surf. Sci. 467–468, 673–683 (2019)
H.H. Adler, P.F. Kerr, Variations in infrared spectra, molecular symmetry and site symmetry of sulfate minerals. Am. Mineral. J. 50, 132–147 (1965)
L.R. Bennedsen, J. Muff, E.G. Søgaard, Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 86, 1092–1097 (2012)
M. Kluska, M. Komasińska, J. Jabłońska, W. Prukała, Challenges of HPLC determination of quinoline derivatives used in the treatment of malaria. J. Liq. Chromatogr. Related Technol. 41, 451–457 (2018)
D. Gupta, R. Chauhan, N. Kumar, V. Singh, V.C. Srivastava, P. Mohanty, T.K. Mandal, Enhancing photocatalytic degradation of quinoline by ZnO:TiO2 mixed oxide: Optimization of operating parameters and mechanistic study. J. Environ. Manage 258, 110032 (2020)
J.R. Bolton, K.G. Bircher, W. Tumas, C.A. Tolman, Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems (IUPAC Technical Report). Pure. Appl. Chem. 73, 627–637 (2001)
O. Pourehie, J. Saien, Treatment of real petroleum refinery wastewater with alternative ferrous-assisted UV/persulfate homogeneous processes. Desalin Water Treat. 142, 140–147 (2019)
US Energy Information Administration (EIA), Independent Statistics and Analysis, US Department of Energy, (2020) Washington DC.
www.Alibaba.com, (2020).
Acknowledgements
The authors would like to acknowledge the Bu-Ali Sina University authorities for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saien, J., Nasri, M. & Pourehie, O. Enhanced activation of persulfate by magnetic CuFe-layered double hydroxide nanocomposites under visible light irradiation for degradation of quinoline. J IRAN CHEM SOC 19, 1515–1526 (2022). https://doi.org/10.1007/s13738-021-02400-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02400-y