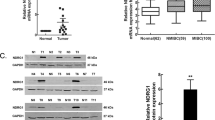
Abstract
Bladder cancer is one of the most prevalent cancers worldwide. Moreover, if not optimally treated, bladder cancer is a significant burden on healthcare systems due to multiple recurrences which often require more aggressive therapies. Therefore, targeted anti-cancer therapies, developed based on an in-depth understanding of specific proteins and molecular mechanisms, are promising in cancer treatment. Here, for the first time, we presented the new approaches indicating that intracellular adhesion molecule-1 (ICAM-1) may play a potential role in enhancing therapeutic effectiveness for bladder cancer. In the present study, we presented that ICAM-1 expression as well as its regulation in bladder cancer is strongly correlated with the high expression of N-cadherin. Importantly, the presence of N-cadherin and its regulator—TWIST-1 was abolished when ICAM-1 was silenced. We identified also that ICAM-1 is capable of regulating cellular migration, proliferation, and EMT progression in bladder cancer cells via the N-cadherin/SRC/AKT/GSK-3β/β-catenin signaling axis. Therefore, we propose ICAM-1 as a novel metastatic marker for EMT progression, which may also be used as a therapeutic target in bladder cancer.









Similar content being viewed by others
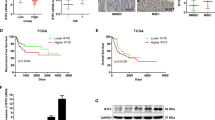
Data availability
Data will be made available on request.
References
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, Guo CC, Lotan Y, Kassouf W. Bladder cancer. Lancet. 2016;3(388):2796–810. https://doi.org/10.1016/S0140-6736(16)30512-8.
Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;13(9):1300. https://doi.org/10.3389/fphar.2018.01300.
Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. https://doi.org/10.1016/s1734-1140(09)70004-0.
Mruk DD, **ao X, Lydka M, Li MW, Bilinska B, Cheng CY. Intercellular adhesion molecule 1: recent findings and new concepts involved in mammalian spermatogenesis. Semin Cell Dev Biol. 2014;29:43–54. https://doi.org/10.1016/j.semcdb.2013.07.003.
Guo P, Huang J, Wang L, Jia D, Yang J, Dillon DA, Zurakowski D, Mao H, Moses MA, Auguste DT. ICAM-1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci USA. 2014;14(111):14710–5. https://doi.org/10.1073/pnas.1408556111.
Usami Y, Ishida K, Sato S, Kishino M, Kiryu M, Ogawa Y, Okura M, Fukuda Y, Toyosawa S. Intercellular adhesion molecule-1 (ICAM-1) expression correlates with oral cancer progression and induces macrophage/cancer cell adhesion. Int J Cancer. 2013;1(133):568–78. https://doi.org/10.1002/ijc.28066.
Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–50. https://doi.org/10.1093/carcin/bgi070.
Tsai ST, Wang PJ, Liou NJ, Lin PS, Chen CH, Chang WC. ICAM1 Is a potential cancer stem cell marker of esophageal squamous cell carcinoma. PLoS ONE. 2015;16(10): e0142834. https://doi.org/10.1371/journal.pone.0142834.
Lim EJ, Kang JH, Kim YJ, Kim S, Lee SJ. ICAM-1 promotes cancer progression by regulating SRC activity as an adapter protein in colorectal cancer. Cell Death Dis. 2022;29(13):417. https://doi.org/10.1038/s41419-022-04862-1.
Gil D, Ciołczyk-Wierzbicka D, Dulińska-Litewka J, Laidler P. Integrin-linked kinase regulates cadherin switch in bladder cancer. Tumour Biol. 2016;37:15185–91. https://doi.org/10.1007/s13277-016-5354-x.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. https://doi.org/10.1038/nrm1835.
Grünert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–65. https://doi.org/10.1038/nrm1175.
Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;15(121):727–35. https://doi.org/10.1242/jcs.000455.
Yang Z, Zhang X, Gang H, Li X, Li Z, Wang T, Han J, Luo T, Wen F, Wu X. Up-regulation of gastric cancer cell invasion by twist is accompanied by n-cadherin and fibronectin expression. Biochem Biophys Res Commun. 2007;6(358):925–30. https://doi.org/10.1016/j.bbrc.2007.05.023.
Gil D, Ciołczyk-Wierzbicka D, Dulińska-Litewka J, Zwawa K, McCubrey JA, Laidler P. The mechanism of contribution of integrin linked kinase (ILK) to epithelial-mesenchymal transition (EMT). Adv Enzyme Regul. 2011;51:195–207. https://doi.org/10.1016/j.advenzreg.2010.09.005.
Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS ONE. 2011;6: e23899. https://doi.org/10.1371/journal.pone.0023899.
Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog. 2011;17(10):5. https://doi.org/10.4103/1477-3163.78111.
Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–20. https://doi.org/10.2353/ajpath.2010.091125.
Maeda K, Kang SM, Sawada T, Nishiguchi Y, Yashiro M, Ogawa Y, Ohira M, Ishikawa T, Hirakawa-YS CK. Expression of intercellular adhesion molecule-1 and prognosis in colorectal cancer. Oncol Rep. 2002;9:511–4.
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W, Ni L. The effect and mechanism of bufalin on regulating hepatocellular carcinoma cell invasion and metastasis via Wnt/β-catenin signaling pathway. Int J Oncol. 2016;48:338–48. https://doi.org/10.3892/ijo.2015.3250.
McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Basecke J, Libra M, Nicoletti F, Cocco L, Martelli AM, Steelman LS. Diverse roles of GSK-3: tumor promoter-tumor suppressor, target in cancer therapy. Adv Biol Regul. 2014;54:176–96. https://doi.org/10.1016/j.jbior.2013.09.013.
Meares GP, Jope RS. Resolution of the nuclear localization mechanism of glycogen synthase kinase-3: functional effects in apoptosis. J Biol Chem. 2007;8(282):16989–7001. https://doi.org/10.1074/jbc.M700610200.
Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001. https://doi.org/10.1083/jcb.200108077.
Goc A, Al-Husein B, Katsanevas K, Steinbach A, Lou U, Sabbineni H, DeRemer DL, Somanath PR. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo. Oncotarget. 2014;15(5):775–87. https://doi.org/10.18632/oncotarget.1770.
Bernardo C, Costa C, Palmeira C, Pinto-Leite R, Oliveira P, Freitas R, Amado F, Santos LL. What we have learned fromurinary bladder cancer models. J Cancer Metastasis Treat. 2016;2:51–8. https://doi.org/10.4103/2394-4722.171279.
Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1:121–2. https://doi.org/10.4161/spmg.1.2.16606.
Ciołczyk-Wierzbicka D, Gil D, Laidler P. The inhibition of cell proliferation using silencing of N-cadherin gene by siRNA process in human melanoma cell lines. Curr Med Chem. 2012;19:145–51. https://doi.org/10.2174/092986712803414006.
Bui TM, Wiesolek HL, Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–99. https://doi.org/10.1002/JLB.2MR0220-549R.
Lekka M, Gil D, Dąbroś W, Jaczewska J, Kulik AJ, Lekki J, Stachura Z, Stachura J, Laidler P. Characterization of N-cadherin unbinding properties in non-malignant (HCV29) and malignant (T24) bladder cells. J Mol Recognit. 2011;24:833–42. https://doi.org/10.1002/jmr.1123.
Gil D, Zarzycka M, Dulińska-Litewka J, Ciołczyk-Wierzbicka D, Lekka M, Laidler P. Dihydrotestosterone increases the risk of bladder cancer in men. Hum Cell. 2019;32:379–89. https://doi.org/10.1007/s13577-019-00255-3.
Lee SH, Wang X, Kim SH, Kim Y, Rodriguez-Puebla ML. Cyclin D3 deficiency inhibits skin tumor development, but does not affect normal keratinocyte proliferation. Oncol Lett. 2017;14:2723–34. https://doi.org/10.3892/ol.2017.6551.
Boonen GJ, van Oirschot BA, van Diepen A, Mackus WJ, Verdonck LF, Rijksen G, Medema RH. Cyclin D3 regulates proliferation and apoptosis of leukemic T cell lines. J Biol Chem. 1999;274:34676–82. https://doi.org/10.1074/jbc.274.49.34676.
Hui L, Zhang S, Dong X, Tian D, Cui Z, Qiu X. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS ONE. 2013;3(8): e62171. https://doi.org/10.1371/journal.pone.0062171.
Fang D, Hawke D, Zheng Y, **a Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;13(282):11221–9. https://doi.org/10.1074/jbc.M611871200.
Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;27(104):5668–73. https://doi.org/10.1073/pnas.0701331104.
Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;18(273):194–200. https://doi.org/10.1016/j.canlet.2008.05.045.
Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;1(12):5074–81. https://doi.org/10.1158/1078-0432.CCR-06-0196.
Wang Z, Liu F, Huang C, Zhang J, Wu J. Bufalin inhibits epithelial-mesenchymal transition and increases radiosensitivity of non-small cell lung cancer via inhibition of the Src signaling. J Thorac Dis. 2023;31(15):123–34. https://doi.org/10.21037/jtd-22-1859.
Liu X, Feng R. Inhibition of epithelial to mesenchymal transition in metastatic breast carcinoma cells by c-Src suppression. Acta Biochim Biophys Sin (Shanghai). 2010;42:496–501. https://doi.org/10.1093/abbs/gmq043.
Pinto-Leite R, Carreira I, Melo J, Ferreira SI, Ribeiro I, Ferreira J, Filipe M, Bernardo C, Arantes-Rodrigues R, Oliveira P, Santos L. Genomic characterization of three urinary bladder cancer cell lines: understanding genomic types of urinary bladder cancer. Tumour Biol. 2014;35:4599–617.
Acknowledgements
This work was supported by the National Science Centre (NCN, Poland, grant no. 2020/04/X/NZ5/00724) and by a grant from Science & Higher Education (MNiSW) through the Jagiellonian University Medical College N41/DBS/000943. The purchase of a microplate reader (BioKom Synergy, HTX) has been supported by a grant from the Priority Research Area qLIFE under the Strategic Programme Excellence Initiative at Jagiellonian University in Kraków. Poland.
Funding
This work was supported by the National Science Centre (NCN, Poland, grant no. 2020/04/X/NZ5/00724) and by a grant from Science & Higher Education (MNiSW) through the Jagiellonian University Medical College N41/DBS/000943. The purchase of a microplate reader (BioKom Synergy, HTX) has been supported by a grant from the Priority Research Area qLIFE under the Strategic Programme Excellence Initiative at Jagiellonian University in Kraków. Poland.
Author information
Authors and Affiliations
Contributions
M.Z. conceived the original idea, designed the study, performed the experiments, performed the figures and graphical abstract, performed statistical analysis of data, and wrote the manuscript; M.K-B. reviewed and edited the manuscript; D.G. performed proliferation analysis, helped with siRNA transfection, and reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zarzycka, M., Kotula-Balak, M. & Gil, D. The mechanism of the contribution of ICAM-1 to epithelial–mesenchymal transition (EMT) in bladder cancer. Human Cell 37, 801–816 (2024). https://doi.org/10.1007/s13577-024-01053-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-024-01053-2