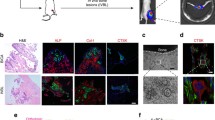
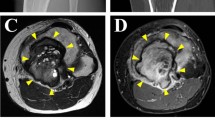
Abstract
The feasibility of a short-term, three-dimensional (3D) culture–based drug sensitivity test (DST) for surgically resected malignant bone tumors, including osteosarcoma (OS), was evaluated utilizing two OS cell line (KCS8 or KCS9)–derived xenograft (CDX) models. Twenty-three (KCS8) or 39 (KCS9) of 60 tested drugs were likely effective in OS cells derived from a cell line before xenografting. Fewer drugs (19: KCS8, 26: KCS9) were selected as effective drugs in cells derived from a CDX tumor, although the drug sensitivities of 60 drugs significantly correlated between both types of samples. The drug sensitivity of a CDX tumor was not significantly altered after the depletion of non-tumorous components in the sample. In a surgically resected metastatic tumor obtained from a patient with OS, for whom a cancer genome profiling test detected a pathogenic PIK3CA mutation, DST identified mTOR and AKT inhibitors as effective drugs. Of two CDX and six clinical samples of OS and Ewing’s sarcoma, DST identified proteasome inhibitors (bortezomib, carfilzomib) and CEP-701 as potentially effective drugs in common. This unique method of in vitro drug testing using 3D-cell cultures is feasible in surgically resected tissues of metastatic malignant bone tumors.




Similar content being viewed by others
References
Fukushima T, Ogura K, Akiyama T, Takeshita K, Kawai A. Descriptive epidemiology and outcomes of bone sarcomas in adolescent and young adult patients in Japan. BMC Musculoskelet Disord. 2018. https://doi.org/10.1186/s12891-018-2217-1.
Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019. https://doi.org/10.1016/j.ejca.2018.11.027.
Kudawara I, Aoki Y, Ueda T, et al. Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide, doxorubicin, cisplatin and high-dose methotrexate in non-metastatic osteosarcoma of the extremities: a phase II trial in Japan. J Chemother. 2013. https://doi.org/10.1179/1973947812Y.0000000055.
Muñoz A, Alfaro J, Pardo N, et al. Long-term results of the Spanish Protocol SO-95 for the treatment of non-metastatic high-grade osteosarcoma of the extremities in children. Clin Transl Oncol. 2009. https://doi.org/10.1007/s12094-009-0373-3.
Lautz TB, Farooqui Z, Jenkins T, et al. Thoracoscopy vs thoracotomy for the management of metastatic osteosarcoma: a pediatric surgical oncology research collaborative study. Int J Cancer. 2021. https://doi.org/10.1002/ijc.33264.
Zoetemelk M, Rausch M, Colin DJ, Dormond O, Nowak-Sliwinska P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-42836-0.
Riedl A, Schlederer M, Pudelko K, et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017. https://doi.org/10.1242/jcs.188102.
Makino H, Nomura S, Kogo H, Wada N, Hayashi M, Yoshida H. Role of collagen gel droplet-embedded culture-drug sensitivity testing (CD-DST) for assessing the sensitivity of gastric cancer to chemotherapy drugs combined with other cancer therapeutic drugs. J Nippon Med Sch. 2022. https://doi.org/10.1272/jnms.JNMS.2022_89-408.
Ariake K, Motoi F, Mizuma M, et al. Collagen gel droplet-embedded culture drug sensitivity test (CD-DST) predicts the effect of adjuvant chemotherapy on pancreatic cancer. Surg Today. 2019. https://doi.org/10.1007/s00595-019-01842-5.
Kii T, Sakuma K, Tanaka A. Optimal contact concentration of paclitaxel in the collagen gel droplet-embedded culture drug sensitivity test for human oral squamous cell carcinoma and evaluation of combination with cetuximab. Chemotherapy. 2021. https://doi.org/10.1159/000512542.
Inoue M, Maeda H, Takeuchi Y, et al. Collagen gel droplet-embedded culture drug sensitivity test for adjuvant chemotherapy after complete resection of non-small-cell lung cancer. Surg Today. 2018. https://doi.org/10.1007/s00595-017-1594-7.
Yoshino Y, Goto H, Ito M, et al. YM155 and chrysin cooperatively suppress survivin expression in SMARCB1/INI1-deficient tumor cells. Med Oncol. 2022. https://doi.org/10.1007/s12032-022-01843-4.
Yokosuka T, Ito M, Yoshino Y, et al. Using the in vitro drug sensitivity test to identify candidate treatments for transient abnormal myelopoiesis. Br J Haematol. 2022. https://doi.org/10.1111/bjh.17970.
Snyder M, Huang XY, Zhang JJ. Identification of novel direct Stat3 target genes for control of growth and differentiation. J Biol Chem. 2008. https://doi.org/10.1074/jbc.M706976200.
Guimarães GM, Tesser-Gamba F, Petrilli AS, et al. Molecular profiling of osteosarcoma in children and adolescents from different age groups using a next-generation sequencing panel. Cancer Genet. 2021. https://doi.org/10.1016/j.cancergen.2021.10.002.
Flobak Å, Skånland SS, Hovig E, Taskén K, Russnes HG. Functional precision cancer medicine: drug sensitivity screening enabled by cell culture models. Trends Pharmacol Sci. 2022. https://doi.org/10.1016/j.tips.2022.08.009.
Wagle MC, Kirouac D, Klijn C, et al. A transcriptional MAPK Pathway Activity Score (MPAS) is a clinically relevant biomarker in multiple cancer types. NPJ Precis Oncol. 2018. https://doi.org/10.1038/s41698-018-0051-4.
Anari F, Ramamurthy C, Zibelman M. Impact of tumor microenvironment composition on therapeutic responses and clinical outcomes in cancer. Future Oncol. 2018. https://doi.org/10.2217/fon-2017-0585.
Shen M, Kang Y. Complex interplay between tumor microenvironment and cancer therapy. Front Med. 2018. https://doi.org/10.1007/s11684-018-0663-7.
Wojtowicz K, Nowicki M. The characterization of the sensitive ovarian cancer cell lines A2780 and W1 in response to ovarian CAFs. Biochem Biophys Res Commun. 2023. https://doi.org/10.1016/j.bbrc.2023.04.059.
Jiang H, Ge H, Shi Y, Yuan F, Yue H. CAFs secrete CXCL12 to accelerate the progression and cisplatin resistance of colorectal cancer through promoting M2 polarization of macrophages. Med Oncol. 2023. https://doi.org/10.1007/s12032-023-01953-7.
Shapovalov Y, Benavidez D, Zuch D, Eliseev RA. Proteasome inhibition with bortezomib suppresses growth and induces apoptosis in osteosarcoma. Int J Cancer. 2010. https://doi.org/10.1002/ijc.25024.
Lu G, Punj V, Chaudhary PM. Proteasome inhibitor bortezomib induces cell cycle arrest and apoptosis in cell lines derived from Ewing’s sarcoma family of tumors and synergizes with TRAIL. Cancer Biol Ther. 2008. https://doi.org/10.4161/cbt.7.4.5564.
Van Stiphout CM, Luu AK, Viloria-Petit AM. Proteasome inhibitors and their potential applicability in osteosarcoma treatment. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14194544.
Antunes BP, Becker RG, Brunetto AT, et al. Expression of neurotrophins and their receptors in primary osteosarcoma. Rev Col Bras Cir. 2019. https://doi.org/10.1590/0100-6991e-20192094.
Heinen TE, Dos Santos RP, da Rocha A, et al. (2016) Trk inhibition reduces cell proliferation and potentiates the effects of chemotherapeutic agents in Ewing sarcoma. Oncotarget. https://doi.org/10.18632/oncotarget.8992
Funding
This work was supported by a Grant for Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED) (20cm0106509h9905).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No authors have conflict of interests to be declared.
Ethical standards
This study has been approved by the ethics committee of Kanagawa Children’s Medical Center (No. 113-8). Written informed consents to be enrolled in the study including cell culture to establish cell lines were obtained from participants in this study or their guardians. Animal experiments have been approved by the ethics committee of Kanagawa Caner Center (No. 04-02 and No. 16-06) and were performed by following Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2023_982_MOESM1_ESM.tif
Supplementary file1 (TIF 144 KB) Supplemental Figure 1. Karyotype analysis of KCS8 and KCS9. Karyotype analysis of osteosarcoma cell lines revealed hyperdiploidy with 66 or 60 chromosomes in KCS8 or KCS9, respectively
13577_2023_982_MOESM2_ESM.tif
Supplementary file2 (TIF 602 KB) Supplemental Figure 2. Immunohistochemistry of KCS8 and KCS9. By immunohistochemistry, both KCS8 and KCS9 were nuclear positive for RUNX2
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goto, H., Ohtsu, T., Ito, M. et al. A short-term three dimensional culture-based drug sensitivity test is feasible for malignant bone tumors. Human Cell 36, 2152–2161 (2023). https://doi.org/10.1007/s13577-023-00982-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00982-8