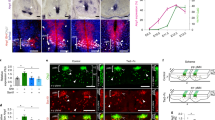
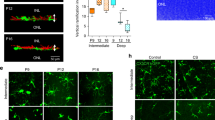
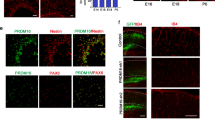
Abstract
Despite the development of neural tissue differentiation methods using a wide variety of stem cells and compartments, there is no standardized strategy for establishing synapses. As the neuronal network is developed in parallel with blood vessel angiogenesis in the central nervous system (CNS) from the embryonic period, we examined neuron–astrocyte–vasculature interactions to understand the effect of the vasculature on the development and stabilization of neurological morphogenesis. We generated a cellular co-culture module targeting the CNS that was embedded in a collagen-based extracellular matrix (ECM) gel. Our neuron–astrocyte–vascular complex module identified the neurological co-localization effect by endothelial cells, as well as the pericyte-induced improvement of synaptic connections. Furthermore, it was suggested that the PDGF, BDNF, IGF, and WNT/BMP pathways were upregulated in synaptic connections enhanced conditions, which are composed of neurexin. These results suggest that the integrity of the vasculature cells in the CNS is important for the establishment of neuronal networks and for synapse connection.




Similar content being viewed by others
References
Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett. 2017;23(652):3–10. https://doi.org/10.1016/j.neulet.2016.12.004.
Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25(6):898–908. https://doi.org/10.1038/s41591-019-0475-6.
Juhasova J, Juhas S, Hruska-Plochan M, et al. Time course of spinal doublecortin expression in develo** rat and porcine spinal cord: implication in in vivo neural precursor grafting studies. Cell Mol Neurobiol. 2015;35(1):57–70. https://doi.org/10.1007/s10571-014-0145-7.
Tai W, Wu W, Wang LL, et al. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell. 2021;28(5):923-937.e4. https://doi.org/10.1016/j.stem.2021.02.009.
Sawada M, Matsumoto M, Sawamoto K. Vascular regulation of adult neurogenesis under physiological and pathological conditions. Front Neurosci. 2014;17(8):53. https://doi.org/10.3389/fnins.2014.00053.
Shin M, Nagai H, Sheng G. Notch mediates Wnt and BMP signals in the early separation of smooth muscle progenitors and blood/endothelial common progenitors. Development. 2009;136(4):595–603. https://doi.org/10.1242/dev.026906.
Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. https://doi.org/10.1038/nature17623.
Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18(3):158–72. https://doi.org/10.1038/s41582-021-00616-3.
Oudega M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012;349(1):269–88. https://doi.org/10.1007/s00441-012-1440-6.
Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12(1):5501. https://doi.org/10.1038/s41467-021-25585-5.
Belayev L, Hong SH, Menghani H, et al. Docosanoids promote neurogenesis and angiogenesis, blood-brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol Neurobiol. 2018;55(8):7090–106. https://doi.org/10.1007/s12035-018-1136-3.
Mostafa S, Pakvasa M, Coalson E, et al. The wonders of BMP9: From mesenchymal stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism to regenerative medicine. Genes Dis. 2019;6(3):201–23. https://doi.org/10.1016/j.gendis.2019.07.003.
Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res. 2020;15(1):16–9. https://doi.org/10.4103/1673-5374.264442.
Hook L, Vives J, Fulton N, et al. Non-immortalized human neural stem (NS) cells as a scalable platform for cellular assays. Neurochem Int. 2011;59(3):432–44. https://doi.org/10.1016/j.neuint.2011.06.024.
McLaren D, Gorba T, de Rotrou AM, et al. Automated large-scale culture and medium-throughput chemical screen for modulators of proliferation and viability of human induced pluripotent stem cell-derived neuroepithelial-like stem cells. J Biomol Screen. 2013;18(3):258–68. https://doi.org/10.1177/1087057112461446.
Conti L, Pollard SM, Gorba T, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3(9):e283. https://doi.org/10.1371/journal.pbio.0030283.
Li S, Zheng J, Chai L, et al. Rapid and efficient differentiation of rodent neural stem cells into oligodendrocyte progenitor cells. Dev Neurosci. 2019;41(1–2):79–93. https://doi.org/10.1159/000499364.
Zhang Y, Lu XY, Casella G, et al. Generation of oligodendrocyte progenitor cells from mouse bone marrow cells. Front Cell Neurosci. 2019;5(13):247. https://doi.org/10.3389/fncel.2019.00247.
Vagaska B, Gillham O, Ferretti P. Modelling human CNS injury with human neural stem cells in 2- and 3-dimensional cultures. Sci Rep. 2020;10(1):6785. https://doi.org/10.1038/s41598-020-62906-y.
Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9(2):168–81. https://doi.org/10.1007/s11481-013-9479-z.
Ma R, **e Q, Li H, et al. l-borneol exerted the neuroprotective effect by promoting angiogenesis coupled with neurogenesis via Ang1-VEGF-BDNF pathway. Front Pharmacol. 2021;12:641894. https://doi.org/10.3389/fphar.2021.641894.
Wu Y, Yang S, Zheng Z, et al. MiR-191–5p disturbed the angiogenesis in a mice model of cerebral infarction by targeting inhibition of BDNF. Neurol India. 2021;69(6):1601–7. https://doi.org/10.4103/0028-3886.333459.
Han W, Jiang L, Song X, Li T, Chen H, Cheng L. VEGF modulates neurogenesis and microvascular remodeling in epileptogenesis after status epilepticus in immature rats. Front Neurol. 2021;12:808568. https://doi.org/10.3389/fneur.2021.808568.
Nieto-Estévez V, Defterali Ç, Vicario-Abejón C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci. 2016;23(10):52. https://doi.org/10.3389/fnins.2016.00052.
Dinsmore CJ, Soriano P. MAPK and PI3K signaling: at the crossroads of neural crest development. Dev Biol. 2018;444(Suppl 1):S79–97. https://doi.org/10.1016/j.ydbio.2018.02.003.
Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;2(4):51. https://doi.org/10.3389/fnmol.2011.00051.
Polevoy H, Gutkovich YE, Michaelov A, Volovik Y, Elkouby YM, Frank D. New roles for Wnt and BMP signaling in neural anteroposterior patterning. EMBO Rep. 2019;20(6):e45842. https://doi.org/10.15252/embr.201845842.
Veit G, Kobbe B, Keene DR, Paulsson M, Koch M, Wagener R. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J Biol Chem. 2006;281(6):3494–504. https://doi.org/10.1074/jbc.M509333200.
Paredes I, Himmels P, de Almodóvar CR. Neurovascular communication during CNS development. Dev Cell. 2018;45(1):10–32. https://doi.org/10.1016/j.devcel.2018.01.023.
Bahney J, von Bartheld CS. The cellular composition and glia-neuron ratio in the spinal cord of a human and a nonhuman primate: comparison with other species and brain regions. Anat Rec (Hoboken). 2018;301(4):697–710. https://doi.org/10.1002/ar.23728.
Liao KH, Chang SJ, Chang HC, et al. Endothelial angiogenesis is directed by RUNX1T1-regulated VEGFA, BMP4 and TGF-β2 expression. PLoS ONE. 2017;12(6):e0179758. https://doi.org/10.1371/journal.pone.0179758.
Jovanovic VM, Salti A, Tilleman H, et al. BMP/SMAD pathway promotes neurogenesis of midbrain dopaminergic neurons in vivo and in human induced pluripotent and neural stem cells. J Neurosci. 2018;38(7):1662–76. https://doi.org/10.1523/JNEUROSCI.1540-17.2018.
Yang L, Liu S, Wang Y. Role of bone morphogenetic protein-2/4 in astrocyte activation in neuropathic pain. Mol Pain. 2019;15:1744806919892100. https://doi.org/10.1177/1744806919892100.
Zhu X, Yan J, Bregere C, et al. RBM3 promotes neurogenesis in a niche-dependent manner via IMP2-IGF2 signaling pathway after hypoxic-ischemic brain injury. Nat Commun. 2019;10(1):3983. https://doi.org/10.1038/s41467-019-11870-x.
De La Fuente AG, Lange S, Silva ME, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017;20(8):1755–64. https://doi.org/10.1016/j.celrep.2017.08.007.
Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci. 2020;12:80. https://doi.org/10.3389/fnagi.2020.00080.
Funding
This work is supported by the foreign Ph.D. students inviting program (2020), The Uehara Memorial Foundation, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The corresponding author Yuzuru Ito belongs to CHIYODA Corporation, Kanagawa, Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moon, S., Ito, Y. Vasculature cells control neuroglial co-localization and synaptic connection in a central nervous system tissue mimic system. Human Cell 36, 1938–1947 (2023). https://doi.org/10.1007/s13577-023-00955-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00955-x