Abstract
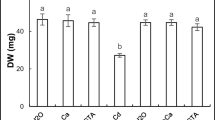
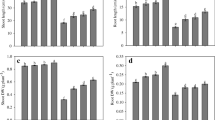
The present investigation aims to evaluate the possible ameliorative role of iron (Fe3+) on cadmium (Cd2+)-induced oxidative stress in mung bean (Vigna radiata L.) seedlings using biochemical and computational approaches. Imposition of Cd2+ stress for a period of 7d showed substantial changes in the growth of root and shoot, and was accompanied by significant Cd2+ accumulation and alteration of biochemical parameters. Cd2+ stress resulted in a significant reduction in root and shoot length, biomass and moisture content, and increased the levels of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide (O2·−). Furthermore, a significant increase in the rate of lipid peroxidation and degradation of antioxidant defence metabolism were observed. Supplementation of the Cd2+-stressed seedlings with Fe3+ for 7d resulted in amelioration of growth, and restoration of normal cellular homeostasis as observed by considerable changes in oxidative stress parameters as compared to Cd2+ treated seedlings alone. Besides, in absence of Fe3+, Cd2+ accumulation in both root and shoot were significantly high as compared to both control and Fe3+-supplemented seedlings. Computational (molecular docking) analysis with superoxide dismutase and catalase as target enzymes revealed that both Cd2+ and Fe3+ have got affinities for the active sites of these antioxidant enzymes. Thus, it is hereby proposed that Cd2+ may potentially interfere with the activities of these antioxidant enzymes, while Fe3+ may prevent such interactions. Our findings based on biochemical and computational analysis clearly reveals the ameliorative role of Fe3+ on Cd2+-induced alterations in growth and oxidative stress in mung bean seedlings.






Similar content being viewed by others
Abbreviations
- AsA:
-
Ascorbate
- BHT:
-
Butylated hydroxytoulene
- CAT:
-
Catalase
- DTNB:
-
5,5′-Dithiobis-2-nitrobenzoic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- GPX:
-
Guaiacol peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- GSSG:
-
Oxidised glutathione
- KSCN:
-
Potassium thiocynate
- MDA:
-
Malondialdehyde
- MVD:
-
Molegro Virtual Docker
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Andresen E, Küpper H (2013) Cadmium toxicity in plants. Cadmium: from toxicity to essentiality. Springer, Dordrecht, pp 395–413
Baxter A, Mittler R, Suzuki N (2014) ROS as a key player in plant stress signalling. J Exp Bot 65:1229–1240
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Method Enzymol 2:764–778
Chen HM, Zheng CR, Tu C, Shen ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Choudhury S, Panda SK (2004) Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol 30:95–110
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signalling in plants under abiotic stress. Plant Signal Behav 8:e23681
Choudhury S, Moulick D, Mazumder MK (2020) Secondary metabolites protect against metal and metalloid stress in rice: an in silico investigation using dehydroascorbate reductase. Acta Physiol Plant. https://doi.org/10.1007/s11738-020-03173-2
Chowardhara B, Borgohain P, Saha B, Awasthi JP, Moulick D, Panda SK (2019) Phytotoxicity of Cd and Zn on three popular Indian mustard varieties during germination and early seedling growth. Biocatal Agric Biotechnol 21:101349
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Elstner EF, Heupel A (1976) Inhibition of nitrate formation from hydroxyl ammonium chloride: a simple assay for superoxide dismutase. Ann Biochem 70:746–778
Faller P, Kienzler K, Liszlay AK (2005) Mechanism of Cd2+ toxicity: Cd2+ inhibited photactivation of photosysyem II by competitive binding to the essential Ca2+ site. Biochem Biophys Acta 1706:158–164
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:8–96
Gill SS, Tuteja NS (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Griffith OW (1980) Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinyl pyridine. Anal Biochem 106:207–211
Halliwell B, Gutteridge JMC (1989) Free radical in biology and medicine. Clerendron Press, Oxford
He XL, Fan SK, Zhu J, Guan MY, Liu XX, Zhang YS, ** CW (2017) Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 416(1–2):453–462
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Iqbal N, Masood A, Nazar R, Syeed S, Khan NA (2010) Photosynthesis, growth and antioxidant metabolism in mustard (Brassica juncea L.) differing in cadmium tolerance. Agric Sci China 9:519–527
Kaznina NM, Titov NF, Topchieva LV, Laidinen GF, Batova YY (2013) Cadmium effect on vacoular H+—ATPase gene expression in roots of barley seedlings of different age. Russ J Plant Physiol 60:55–59
Lagriffoul A, Mocquot B, Mench M, Vangronsveld J (1998) Cadmium toxicity effects on growth and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 200(2):241–250
Li X, Gong B, Xu K (2014) Interaction of nitric oxide and polyamines involves antioxidants and physiological strategies against chilling-induced oxidative damage in Zingiber officinale Roscoe. Sci Hortic 170:237–248
Liang Y, Wong J, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Lozano-Rodriguez E, Harnandez LE, Bonay P, Carpena-Ruiz RO (1997) Distribution of cadmium in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48:123–128
Lux A, Martinka M, Vacul´ık M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Moulick D, Chowardhara B, Panda SK (2019) Agroecotoxicological aspect of arsenic (As) and cadmium (Cd) on field crops and its mitigation: current status and future prospect. Plant–metal Interactions. Springer, Cham, pp 217–246
Muneer S, Kim TH, Qureshi MI (2012) Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata. Plant Growth Regul 68(3):421–433
Muneer S, Ahmad J, Qureshi M (2013) Involvement of Fe nutrition in modulating oxidative stress and the expression of stress responsive proteins in leaves of Vigna radiata L. Aust J Crop Sci 7(9):1333
Nagalakshmi N, Prasad MNV (2000) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Ortega-Villasante C, Harnendez LE, Rellen-Alvarez R, del Campo FF, Carpena-Ruiz RO (2009) Rapid alteration of cellular redox homeostasis upon exposure to cadmium and mercury in alfalfa seedlings. New Phytol 176:96–107
Oser B (1979) Hawks physiological chemistry. McGraw-Hill, New York
Piqueras A, Olmos E, Martinez-Solano JR, Hellin E (1999) Cadmium induced oxidative burst in tobacco BY2 cells: time course, subcellular localization and antioxidant response. Free Radic Res 33:8
Qureshi MI, Qadir S, Zolla L (2007) Proteomics-based dissection of stress-responsive pathways in plants. J Plant Physiol 164:1239–1260
Rodriguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, del Rio LA, Sandalio LM (2006) Cadmium effects on oxidative metabolism in pea (Pisum sativum L.) roots: Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Sagisaka S (1979) The occurrence of peroxide in a perennial plant, Populus gelrica. Plant Physiol 57:308–309
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sanita di Toppi L, Gibbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Schutzendubel A, Schwan P, Teichmann T, Gross K, Langefeld-Heyser R, Godbold DL, Polle A (2001) Cadmium induced changes in antioxidant system, hydrogen peroxide content and differentiation in scot pine (Pinus sylvestris) roots. Plant Physiol 127:887–892
Seregin IV, Ivanov VB (2001) Physiological aspects of cadmium and lead toxic effects on higher plants. Russ J Plant Physiol 48(4):523–544
Seth CS, Misra V, Chauhan LKS, Singh RR (2008) Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and Comet assay approach. Ecotoxicol Environ Saf 71:711–716
Shah K, Kumar RG, Verma RS, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shao G, Chen M, Wang W, Mou R (2007) Iron nutrition affects cadmium accumulation and toxicity in rice plants. Plant Growth Regul 53(1):33–42
Shi X, Zhang C, Wang H, Zhnag F (2005) Effect of Si on distribution of Cd in rice seedlings. Plant Soil 272:53–60
Shi G, Cai Q, Lui C, Wu L (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and simulation of antioxidant enzymes. Plant Growth Regul 61:45–52
Smith IK, Vierhgeller TL, Throne CA (1988) Assay of glutathione reductase in crude tissue homogenate using 5,5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Verma K, Shekhawat GS, Sharma A, Mehta SK, Sharma V (2009) Cadmium induced oxidative stress and changes in soluble and ionically bound cell wall peroxidase activity in roots of seedling and 3–4 leaf stage plant of Brassica juncea (L.) czern. Plant Cell Rep 27(7):1261–1269
Wang H, Wang T, Ahmad I (2015) Involvement of phosphate supplies in different transcriptionally regulated pathway of Oryza sativa L’s antioxidative system in response to arsenite and cadmium stress. Ecotoxicology 24:1259–1268
Zhang C, Wang L, Nei Q, Zhang W, Zhang F (2008) Long term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ Exp Bot 62:300–307
Acknowledgements
The authors sincerely acknowledges Assam University, Silchar for providing all necessary facilities for this study.
Author information
Authors and Affiliations
Contributions
SC designed, supervised the study, performed the biochemical analysis and wrote part of the manuscript. MKJ and DM conducted the computational (docking) analysis and wrote major part of the manuscript (equal contribution).
Corresponding author
Ethics declarations
Conflict of interest
All author declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazumder, M.K., Moulick, D. & Choudhury, S. Iron (Fe3+)-mediated redox responses and amelioration of oxidative stress in cadmium (Cd2+) stressed mung bean seedlings: a biochemical and computational analysis. J. Plant Biochem. Biotechnol. 31, 49–60 (2022). https://doi.org/10.1007/s13562-021-00654-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-021-00654-4