Abstract
Objective
The ecotoxic effects of pesticide used for mosquito’s control TRISADA® (TRI) [deltamethrin (D) 1%+tetramethrin (T) 0.33%, and piperonyl butoxide (PB) 0.29%] on amphibian larvae were investigated.
Methods
In the laboratory, Rhinella arenarum tadpoles were exposed to nominal concentrations of 0.0000 (control; CO), 0.0003125% (C1); 0.000625% (C2); 0.00125% (C3); 0.0025% (C4); 0.005% (C5) (v/v) of formulated TRI. Median lethal concentration (LC50) (%) and 95% confidence limits (CL), the no-observedeffect concentration (NOEC), and the lowest-observedeffect concentration (LOEC) were quantified. The possible effects of TRI on B-esterases, evaluated through acetylcholinesterase (AChE) and carboxylesterase (CbE) activities, in addition to swimming performance (distance moved, mean speed, maximum speed, global activity, and resting time or immobility) were measured in tadpoles whose concentrations displayed survival rates higher than 50%.
Results
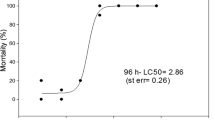
The 48 h LC50 of TRI was 0.00125% (v/v) [12.5 (D)+4.1 (T)+3.6 (PB); μg L-1] (CL: 0.000811- 0.001926%). The 48 h NOEC and LOEC values were 0.0003125% (v/v) [3.1 (D)+1 (T)+0.9 (PB); μg L-1] and 0.000625% (v/v) [6.2 (D) +2 (T) +1.8 (PB); μg L-1], respectively. At 48 h of exposure to upper sublethal TRI concentration assay (C3), AChE and CbE activities were significantly inhibited (68 and 84%, respectively) with respect to controls. Also, all the sublethal TRI concentrations caused significantly alterations of all swimming endpoints evaluated.
Conclusion
The current study established that pesticide TRI is toxic to R. arenarum tadpoles and had detrimental effects on the B-sterases activities and swimming activity at TRI sublethal concentrations.
Similar content being viewed by others
References
Hayes, T. B., Falso, P., Gallipeau, S. & Stice, M. The cause of global amphibian declines: a developmental endocrinologist’s perspective. J. Exp. Biol. 213, 921–933 (2010).
Sparling, D. W., Linder, G., Bishop, C. A. & Krest, S. K. in Ecotoxicology of Amphibians and Reptiles, 2nd edn (eds Sparling, D. W., Linder, G., Bishop, C. A. & Krest, S. K.) 1–11 (CRC Press, Taylor & Francis Group, New York, 2010).
Hemingway, J. The role of vector control in stop** the transmission of malaria: threats and opportunities. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 369, doi: 10.1098/ rstb.2013.0431 (2014).
WHO. Fifteenth report of the WHO Expert Committee on Vector Biology and Control, http://apps.who.int/ iris/bitstream/handle/10665/37432/WHO_TRS_818. pdf?sequence=1&isAllowed=y (1992).
Rehman, H. et al. Systematic review on pyrethroid toxicity with special reference to deltamethrin. J. Entomol. Zool. Stud. 2, 60–70 (2014).
Cakir, G., Yavuz, O. & Kocak, O. Effects of piperonyl butoxide and tetramethrin combinations on biological activities of selected synthetic pyrethroid insecticides against different Housefly (Musca domestica L., Diptera: Muscidae) populations. Acta. Vet. Brno. 77, 467–474 (2008).
Merivee, E. et al. Low doses of the common alpha–cypermethrin insecticide affect behavioural thermoregulation of the non–targeted, beneficial carabid beetle Platynus assimilis (Coleoptera: Carabidae). Ecotoxicol. Environ. Saf. 120, 286–294 (2015).
Berrill, M. et al. Lethal and sublethal impacts of pyrethroid insecticides on amphibian embryos and tadpoles. Environ. Toxicol. Chem. 12, 525–539 (1993).
Sánchez–Hernández, J. C. in Environmental pollution: new research (eds Plattenberg, R. H.) 1–45 (Nova, New York, 2006).
Freitas, J. S., Felício, A. A., Teresa, F. B. & Alves de Almeida, E. Combined effects of temperature and clomazone (Gamit®) on oxidative stress responses and B–esterase activity of Physalaemus nattereri (Leiuperidae) and Rhinella schneideri (Bufonidae) tadpoles. Chemospher. 185, 548–562 (2017).
Wheelock, C. E. et al. Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev. Environ. Contam. Toxicol. 195, 117–178 (2008).
Peltzer, P. et al. Effect of exposure to contaminated pond sediments on survival, development, and enzyme and blood biomarkers in veined treefrog (Trachycephalus typhonius) tadpoles. Ecotoxicol. Environ. Saf. 98, 142–151 (2013).
Attademo, A. M., Lajmanovich, R. C., Peltzer, P. M. & Junges, C. Acute toxicity of metaldehyde in the invasive rice snail Pomacea canaliculata and sublethal effects on tadpoles of a non–target species (Rhinella arenarum). Water Air Soil Pollut. 227, 1–12 (2016).
Robles–Mendoza, C. et al. Esterases activity in the axolotl Ambystoma mexicanum exposed to chlorpyrifos and its implication to motor activity. Aquat. Toxicol. 105, 728–734 (2011).
Denoël, M. et al. Effects of a sublethal pesticide exposure on locomotor behavior: a video–tracking analysis in larval amphibians. Chemospher. 90, 945–951 (2013).
Egea–Serrano, A. & Tejedo, M. Contrasting effects of nitrogenous pollution on fitness and swimming performance of Iberian water frog, Pelophylax perezi (Seoane, 1885), larvae in mesocosms and field enclosures. Aquat. Toxicol. 146, 144–153 (2014).
Junges, C. M. et al. Acute toxicity and etho–toxicity of three insecticides used for mosquitoes control on amphibian tadpoles. Water. Air. Soil. Pollut. 228, 143–153 (2017).
Hemingway, J. & Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45, 371–391 (2000).
Wu, X. M. et al. Identification of carboxylesterase genes associated with pyrethroid resistance in the malaria vector Anopheles sinensis (Diptera: Culicidae). Pest. Manag. Sci. 74, 159–169 (2018).
Salibián, A. Effects of deltametrhin on the south american toad (Bufo arenarum). Bull. Environ. Contam. Toxicol. 48, 616–621 (1992).
de Knecht, J. A. & van Herwijnen, R. Environmental risk limits for deltamethrin. https://www.researchgate. net/publication/237125551_Environmental_risk_limits_ for_deltamethrin.pdf (2008).
Aydin–Sinan, H., Güngördü, A. & Ozmen, M. Toxic effects of deltamethrin and ë–cyhalothrin on Xenopus laevis tadpoles. J. Environ. Sci. Health B. 47, 397–402 (2012).
Macagnan, N. et al. Toxicity of cypermethrin and deltamethrin insecticides on embryos and larvae of Physalaemus gracilis (Anura: Leptodactylidae). Environ. Sci. Pollut. Res. Int. 24, 20699–20704 (2017).
Zhang, Z. Y. et al. Acute toxicity to zebrafish of two organophosphates and four pyrethroids and their binary mixtures. Pest. Manag. Sci. 66, 84–89 (2010).
Conney, A. H. et al. Effects of piperonyl butoxide on drug metabolism in rodents and man. Arch. Environ. Occup. Healt. 24, 97–106 (1972).
Wickham, J. in Piperonyl Butoxide: the insecticide synergist (eds Jones, D. G.) 239–260 (Academic Press, London, 1998).
Sánchez–Bayo, F. Insecticides Mode of Action in Relation to Their Toxicity to Non–Target Organisms. J. Environment. Analytic. Toxicol. S4, doi:10.4172/2161–0525. S4–002 (2012).
Schleier, J. J. & Peterson R. K. D. The Joint Toxicity of Type I, II,and Nonester Pyrethroid Insecticides. J. Econ. Entomol. 105, 85–91 (2012).
Spitzer, N. C. & Borodinsky, L. N. Implications of activity–dependent neurotransmitter—receptor matching. Philos. Trans. R. Soc. Lond., B., Biol. Sci. 363, doi: 10. 1098/rstb.2007.2257 (2008).
Das, B. K. & Mukherjee, S. C. Chronic toxic effects of quinalphos on some biochemical parameters in Labeo rohita (Ham.). Toxicol. Lett. 114, 11–18 (2000).
Velisek, J. et al. Effects of deltamethrin on rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Pharmacol. 23, 297–301 (2007).
Tu, H. T. et al. Combined effects of deltamethrin, tem perature and salinity on oxidative stress biomarkers and acetylcholinesterase activity in the black tiger shrimp (Penaeus monodon). Chemospher. 86, 83–91 (2012).
Üner, N., Piner, P. & Temiz, Ö. Piperonyl butoxide increases oxidative toxicity of fenthion in the brain of Oreochromis niloticus. J. Biochem. Mol. Toxicol. 28, 84–90 (2014).
Maxwell, D. M. The specificity of carboxylesterase protection against the toxicity of organophosphate compounds. Toxicol. Appl. Pharmacol. 114, 306–312 (1992).
Denton, D. L. Joint acute toxicity of esfenvalerate and diazinon to fathead minnow (Pimephales promelas) larvae. Environ. Toxicol. Chem. 22, 336–341 (2003).
David, M., Marigoudar, S. R., Patil, V. K. & Halappa, R. Behavioral, morphological deformities and biomarkers of oxidative damage as indicators of sublethal cypermethrin intoxication on the tadpoles of D. melanostictus (Schneider, 1799). Pest. Biochem. Physiol. 103, 127–134 (2012).
Materna, E. J., Rabeni, C. F. & Lapoint, T. W. Effects of the synthetic pyrethroid insecticide, esfenvalerate, on larval leopard frogs (Rana spp.). Environ. Toxicol. Chem. 14, 613–622 (1995).
Agostini, M. G., Natale, G. S. & Ronco, A. E. Lethal and sublethal effects of cypermethrin to Hypsiboas pulchellus tadpoles. Ecotoxicolog. 19, 1545–1550 (2010).
Casco, V. et al. Apoptotic cell death in the central nervous system of Bufo arenarum tadpoles induced by cypermethrin. Cell Biol. Toxicol. 22, 199–211 (2006).
Mushigeri, S. & David, M. Fenvalerate induced changes in the Ach and associated AchE activity in different tissues of fish Cirrhinus mrigala (Hamilton) under lethal and sub–lethal exposure period. Environ. Toxicol. Pharmacol. 20, 65–72 (2005).
Marigoudar, S. R., Nazeer Ahmed, R. & David, M. Impact of cypermethrin on Behavioural responses in the fresh water teleost, Labeo rohita (Hamilton). World. J. Zool. 4, 19–23 (2009).
Gan, J. et al. Distribution and persistence of pyrethroids in runoff sediments. J. Environ. Qual. 34, 836–841 (2005).
Gosner, K. L. A simplified table for staging anuran embryos and larvae, with notes on identification. Herpetologic. 16, 183–190 (1960).
ASIH: American Society of Ichthyologists and Herpetologists. Guidelines for use of live amphibians and reptiles in field and laboratory research, 2nd edn (Herpetological Animal Care and Use Committee (HACC) of the American Society of Ichthyologists and Herpetologists, USA, 2004).
Schleier, J. J. & Peterson, R. K. D. Toxicity and risk of permethrin and naled to non–target insects after adult mosquito management. Ecotoxicol. 19, 1140–1146 (2010).
Kingsley, G. R. The direct biuret method for the determination of serum proteins as applied to photoelectric and visual colorimetry. J. Lab. Clin. Med. 27, 840–845 (1942).
Ellman, G. L., Courtney, K. D., Andreas, V. Jr. & Featherstone, R. M. A new and rapid calorimetric determination of cholinesterase activity. Biochem. Pharmacol. 7, 88–95 (1961).
Bunyan, P. J. & Jennings, D. M. Organophosphorus poisoning; some properties of avian esterase. J. Lab. Clin. Med. 16, 326–331 (1968).
Hamilton, M. A., Russo, R. C. & Thurston, R. V. Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Tech. 11, 714–719 (1977).
Ayres, M. Jr., Ayres, D. & Santos, A. BioEstat, Versao 5.0. Sociedade Civil Mamirauá, MCT–CNPq, https:// www.mamiraua.org.br/pt–br/downloads/programas/ bioestat–versao–53/ (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lajmanovich, R.C., Peltzer, P.M., Martinuzzi, C.S. et al. B-esterases and Behavioral Biomarkers in Tadpoles Exposed to Pesticide Pyrethroid-TRISADA®. Toxicol. Environ. Health Sci. 10, 237–244 (2018). https://doi.org/10.1007/s13530-018-0371-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-018-0371-3